Lopinavir/r no longer recommended as a first-line regimen: a comparative effectiveness analysis
- PMID: 25261780
- PMCID: PMC4176690
- DOI: 10.7448/IAS.17.1.19070
Lopinavir/r no longer recommended as a first-line regimen: a comparative effectiveness analysis
Abstract
Introduction: We compared the effectiveness of tenofovir/emtricitabine (TDF/FTC) combined with either lopinavir/r (LPV/r) or another recommended third drug in the 2010 French guidelines in antiretroviral-naïve patients starting combination antiretroviral therapy in 2004-2008 in the French Hospital Database on HIV.
Methods: The outcomes were stop or switch of the third component, viral load (VL) <500 copies/ml, an increase of at least 100 CD4 cells/mm(3), AIDS-defining event and non-AIDS-defining hospitalization or death. Propensity scores were estimated by logistic regression based on the clinical centre and other confounders. In each clinical centre, each patient initiating LPV/r was matched with a patient initiating another third drug (efavirenz or atazanavir/r) and having a close propensity score. Cox's proportional hazards models were then used, with treatment as covariate. Time was right-censored at four years.
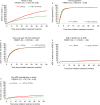
Results: 1269 patients started LPV/r plus TDF/FTC, and 890 could be matched to 890 patients receiving another third drug. Baseline characteristics were well balanced between these two groups. LPV/r was associated with a higher risk of third drug stop (hazard ratio (HR): 1.69; 95% confidence interval (CI), 1.42-2.00) and with less rapid viral suppression (HR: 0.83; 95% CI, 0.72-0.95). There was no difference in the time required for a CD4 cell increment of at least 100/mm(3), or to the occurrence of an AIDS-defining event. Non-AIDS-defining hospitalizations or deaths were more frequent with LPV/r (HR: 1.79; 95% CI, 1.33-2.39).
Conclusions: For first-line therapy, in this observational setting, TDF/FTC plus LPV/r were less durable than TDF/FTC plus another recommended third drug, led to a less rapid viral suppression and were associated with a higher risk of non-AIDS morbidity.
Keywords: HIV infection; antiretroviral therapy; effectiveness; first-line therapy; propensity score.
Figures

References
-
- Elion R, Green L, Cohen C, Green S, Baird I, Schrader S, et al. An open-label trial of stavudine, lamivudine and efavirenz in the treatment of HIV-positive, treatment-naive patients, and implications for clinical practice. Antivir Ther. 1999;4(Suppl 3):S89–91. - PubMed
-
- Hicks C, King M, Gulick R, Eron J, Clinton White A, Kessler H, et al. Long-term safety and durable antiretroviral activity of lopinavir/ritonavir in treatment-naive patients: 4 years follow-up study. AIDS. 2004;18:775–9. - PubMed
-
- Molina JM, Andrade-Villanueva J, Echevarria J, Chetchotisakd P, Corral J, David N, et al. Once-daily atazanavir/ritonavir versus twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 48 week efficacy and safety results of the CASTLE study. Lancet. 2008;372:646–55. - PubMed
-
- Molina JM, Andrade-Villanueva J, Echevarria J, Chetchotisakd P, Corral J, David N, et al. Once-daily atazanavir/ritonavir compared with twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1 infected patients: 96-week efficacy and safety results of the CASTLE study. J Acquir Immune Defic Syndr. 2010;53(3):323–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

