Molecular profiling of circulating tumor cells links plasticity to the metastatic process in endometrial cancer
- PMID: 25261936
- PMCID: PMC4190574
- DOI: 10.1186/1476-4598-13-223
Molecular profiling of circulating tumor cells links plasticity to the metastatic process in endometrial cancer
Abstract
Background: About 20% of patients diagnosed with endometrial cancer (EC) are considered high-risk with unfavorable prognosis. In the framework of the European Network for Individualized Treatment in EC (ENITEC), we investigated the presence and phenotypic features of Circulating Tumor Cells (CTC) in high-risk EC patients.
Methods: CTC isolation was carried out in peripheral blood samples from 34 patients, ranging from Grade 3 Stage IB to Stage IV carcinomas and recurrences, and 27 healthy controls using two methodologies. Samples were subjected to EpCAM-based immunoisolation using the CELLection™ Epithelial Enrich kit (Invitrogen, Dynal) followed by RTqPCR analysis. The phenotypic determinants of endometrial CTC in terms of pathogenesis, hormone receptor pathways, stem cell markers and epithelial to mesenchymal transition (EMT) drivers were asked. Kruskal-Wallis analysis followed by Dunn's post-test was used for comparisons between groups. Statistical significance was set at p < 0.05.
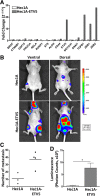
Results: EpCAM-based immunoisolation positively detected CTC in high-risk endometrial cancer patients. CTC characterization indicated a remarkable plasticity phenotype defined by the expression of the EMT markers ETV5, NOTCH1, SNAI1, TGFB1, ZEB1 and ZEB2. In addition, the expression of ALDH and CD44 pointed to an association with stemness, while the expression of CTNNB1, STS, GDF15, RELA, RUNX1, BRAF and PIK3CA suggested potential therapeutic targets. We further recapitulated the EMT phenotype found in endometrial CTC through the up-regulation of ETV5 in an EC cell line, and validated in an animal model of systemic dissemination the propensity of these CTC in the accomplishment of metastasis.
Conclusions: Our results associate the presence of CTC with high-risk EC. Gene-expression profiling characterized a CTC-plasticity phenotype with stemness and EMT features. We finally recapitulated this CTC-phenotype by over-expressing ETV5 in the EC cell line Hec1A and demonstrated an advantage in the promotion of metastasis in an in vivo mouse model of CTC dissemination and homing.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

