Histone deacetylase 3 (HDAC3) plays an important role in retinal ganglion cell death after acute optic nerve injury
- PMID: 25261965
- PMCID: PMC4190472
- DOI: 10.1186/1750-1326-9-39
Histone deacetylase 3 (HDAC3) plays an important role in retinal ganglion cell death after acute optic nerve injury
Abstract
Background: Optic nerve damage initiates a series of early atrophic events in retinal ganglion cells (RGCs) that precede the BAX-dependent committed step of the intrinsic apoptotic program. Nuclear atrophy, including global histone deacetylation, heterochromatin formation, shrinkage and collapse of nuclear structure, and the silencing of normal gene expression, comprise an important obstacle to overcome in therapeutic approaches to preserve neuronal function. Several studies have implicated histone deacetylases (HDACs) in the early stages of neuronal cell death, including RGCs. Importantly, these neurons exhibit nuclear translocation of HDAC3 shortly after optic nerve damage. Additionally, HDAC3 activity has been reported to be selectively toxic to neurons.
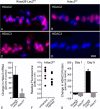
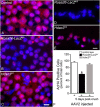
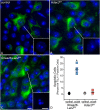
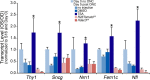
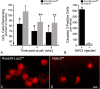
Results: RGC-specific conditional knockout of Hdac3 was achieved by transducing the RGCs of Hdac3fl/fl mice with an adeno-associated virus serotype 2 carrying CRE recombinase and GFP (AAV2-Cre/GFP). Controls included similar viral transduction of Rosa26fl/fl reporter mice. Optic nerve crush (ONC) was then performed on eyes. The ablation of Hdac3 in RGCs resulted in significant amelioration of characteristics of ONC-induced nuclear atrophy such as H4 deacetylation, heterochromatin formation, and the loss of nuclear structure. RGC death was also significantly reduced. Interestingly, loss of Hdac3 expression did not lead to protection against RGC-specific gene silencing after ONC, although this effect was achieved using the broad spectrum inhibitor, Trichostatin A.
Conclusion: Although other HDACs may be responsible for gene expression changes in RGCs, our results indicate a critical role for HDAC3 in nuclear atrophy in RGC apoptosis following axonal injury. This study provides a framework for studying the roles of other prevalent retinal HDACs in neuronal death as a result of axonal injury.
Figures




References
-
- Quigley HA, Nickells RW, Kerrigan LA, Pease ME, Thibault DJ, Zack DJ. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci. 1995;36(5):774–786. - PubMed
-
- Fortune B, Burgoyne CF, Cull GA, Reynaud J, Wang L. Structural and functional abnormalities of retinal ganglion cells measured in vivo at the onset of optic nerve head surface change in experimental glaucoma. Invest Ophthalmol Vis Sci. 2012;53(7):3939–3950. doi: 10.1167/iovs.12-9979. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

