Effects of β-sitosterol derived from Artemisia capillaris on the activated human hepatic stellate cells and dimethylnitrosamine-induced mouse liver fibrosis
- PMID: 25262005
- PMCID: PMC4193130
- DOI: 10.1186/1472-6882-14-363
Effects of β-sitosterol derived from Artemisia capillaris on the activated human hepatic stellate cells and dimethylnitrosamine-induced mouse liver fibrosis
Abstract
Background: β-sitosterol is a cholesterol-like phytosterol, which widely distributed in the plant kingdom. Here, anti-fibrotic effect of the β-sitosterol was studied using the activated human hepatic stellate cell (HSC) model and dimethylnitrosamine (DMN)-induced mouse hepatic fibrosis model.
Method: HSCs were activated by transforming growth factor-β (TGF-β) and the collagen-1 and α-smooth muscle actin (α-SMA) expressions were measured at the mRNA and protein level. We also studied the effect β-sitosterol using DMN-induced mouse hepatic fibrosis model. We then measured the collagen-1 and α-SMA expression levels in vivo to investigate anti-hepatofibrotic effect of β-sitosterol, at both of the mRNA and protein level.
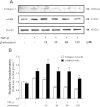
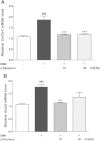
Results: β-sitosterol down regulated the mRNA and protein expression levels of collagen-1 and α-SMA in activated HSC. Oral administration of the β-sitosterol successfully alleviated the DMN-induced mouse liver damage and prevented collagen accumulation. The mRNA and protein expression levels of collagen-1 and α-SMA were also down regulated in β-sitosterol treated mouse group.
Conclusions: This study shows the effect of β-sitosterol on the TGF-β -or DMN-induced hepatofibrosis. Hence, we demonstrate the β-sitosterol as a potential therapeutic agent for the hepatofibrosis.
Figures






Similar articles
-
Fibroblast growth factor 21 attenuates hepatic fibrogenesis through TGF-β/smad2/3 and NF-κB signaling pathways.Toxicol Appl Pharmacol. 2016 Jan 1;290:43-53. doi: 10.1016/j.taap.2015.11.012. Epub 2015 Nov 22. Toxicol Appl Pharmacol. 2016. PMID: 26592322
-
Synergistic anti-liver fibrosis actions of total astragalus saponins and glycyrrhizic acid via TGF-β1/Smads signaling pathway modulation.J Ethnopharmacol. 2016 Aug 22;190:83-90. doi: 10.1016/j.jep.2016.06.011. Epub 2016 Jun 6. J Ethnopharmacol. 2016. PMID: 27282665
-
A Chinese herbal medicine, Gexia-Zhuyu Tang (GZT), prevents dimethylnitrosamine-induced liver fibrosis through inhibition of hepatic stellate cells proliferation.J Ethnopharmacol. 2012 Aug 1;142(3):811-8. doi: 10.1016/j.jep.2012.06.005. Epub 2012 Jun 15. J Ethnopharmacol. 2012. PMID: 22706148
-
Multifunctional roles and pharmacological potential of β-sitosterol: Emerging evidence toward clinical applications.Chem Biol Interact. 2022 Sep 25;365:110117. doi: 10.1016/j.cbi.2022.110117. Epub 2022 Aug 19. Chem Biol Interact. 2022. PMID: 35995256 Review.
-
Critical Analysis on Characterization, Systemic Effect, and Therapeutic Potential of Beta-Sitosterol: A Plant-Derived Orphan Phytosterol.Medicines (Basel). 2016 Nov 15;3(4):29. doi: 10.3390/medicines3040029. Medicines (Basel). 2016. PMID: 28930139 Free PMC article. Review.
Cited by
-
Mechanisms of Xiaochaihu Decoction on Treating Hepatic Fibrosis Explored by Network Pharmacology.Dis Markers. 2022 Oct 4;2022:8925637. doi: 10.1155/2022/8925637. eCollection 2022. Dis Markers. 2022. PMID: 36246566 Free PMC article.
-
The stem bark decoction of Myrianthus arboreus P. Beauv. (Cecropiaceae) shows anti-uterine leiomyoma effects in Wistar rat.Reprod Fertil. 2025 Jul 26;6(3):e250037. doi: 10.1530/RAF-25-0037. Print 2025 Jul 1. Reprod Fertil. 2025. PMID: 40626852 Free PMC article.
-
Preventive effects of total saponins of Panax japonicus on fatty liver fibrosis in mice.Arch Med Sci. 2018 Mar;14(2):396-406. doi: 10.5114/aoms.2016.63260. Epub 2016 Oct 26. Arch Med Sci. 2018. PMID: 29593815 Free PMC article.
-
A Survey of Therapeutic Effects of Artemisia capillaris in Liver Diseases.Evid Based Complement Alternat Med. 2015;2015:728137. doi: 10.1155/2015/728137. Epub 2015 Aug 20. Evid Based Complement Alternat Med. 2015. PMID: 26366183 Free PMC article. Review.
-
Puerarin: a hepatoprotective drug from bench to bedside.Chin Med. 2024 Oct 8;19(1):139. doi: 10.1186/s13020-024-01011-y. Chin Med. 2024. PMID: 39380120 Free PMC article. Review.
References
-
- Poynard T, Mathurin P, Lai CL, Guyader D, Poupon R, Tainturier MH, Myers RP, Muntenau M, Ratziu V, Manns M, Vogel A, Capron F, Chedid A, Bedossa P, Panfibrosis Group A comparison of fibrosis progression in chronic liver diseases. J Hepatol. 2003;38(3):257–265. doi: 10.1016/S0168-8278(02)00413-0. - DOI - PubMed
Pre-publication history
-
- The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1472-6882/14/363/prepub
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

