XTACC3-XMAP215 association reveals an asymmetric interaction promoting microtubule elongation
- PMID: 25262927
- PMCID: PMC4200520
- DOI: 10.1038/ncomms6072
XTACC3-XMAP215 association reveals an asymmetric interaction promoting microtubule elongation
Abstract
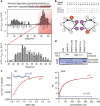
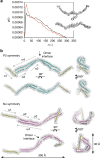
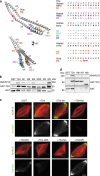
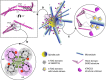
chTOG is a conserved microtubule polymerase that catalyses the addition of tubulin dimers to promote microtubule growth. chTOG interacts with TACC3, a member of the transforming acidic coiled-coil (TACC) family. Here we analyse their association using the Xenopus homologues, XTACC3 (TACC3) and XMAP215 (chTOG), dissecting the mechanism by which their interaction promotes microtubule elongation during spindle assembly. Using SAXS, we show that the TACC domain (TD) is an elongated structure that mediates the interaction with the C terminus of XMAP215. Our data suggest that one TD and two XMAP215 molecules associate to form a four-helix coiled-coil complex. A hybrid methods approach was used to define the precise regions of the TACC heptad repeat and the XMAP215 C terminus required for assembly and functioning of the complex. We show that XTACC3 can induce the recruitment of larger amounts of XMAP215 by increasing its local concentration, thereby promoting efficient microtubule elongation during mitosis.
Figures


References
-
- Al-Bassam J., Larsen N. A., Hyman A. A. & Harrison S. C. Crystal structure of a TOG domain: conserved features of XMAP215/Dis1-family TOG domains and implications for tubulin binding. Structure 15, 355–362 (2007). - PubMed
-
- Reber S. B. et al.. XMAP215 activity sets spindle length by controlling the total mass of spindle microtubules. Nat. Cell. Biol. 15, 1116–1122 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

