Engineering a vascularized collagen-β-tricalcium phosphate graft using an electrochemical approach
- PMID: 25263031
- PMCID: PMC4525774
- DOI: 10.1016/j.actbio.2014.09.035
Engineering a vascularized collagen-β-tricalcium phosphate graft using an electrochemical approach
Abstract
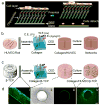
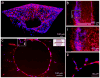
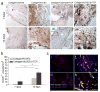
Vascularization of three-dimensional large synthetic grafts for tissue regeneration remains a significant challenge. Here we demonstrate an electrochemical approach, named the cell electrochemical detachment (CED) technique, to form an integral endothelium and use it to prevascularize a collagen-β-tricalcium phosphate (β-TCP) graft. The CED technique electrochemically detached an integral endothelium from a gold-coated glass rod to a collagen-infiltrated, channeled, macroporous β-TCP scaffold, forming an endothelium-lined microchannel containing graft upon removal of the rod. The in vitro results from static and perfusion culture showed that the endothelium robustly emanated microvascular sprouting and prevascularized the entire collagen/β-TCP integrated graft. The in vivo subcutaneous implantation studies showed that the prevascularized collagen/β-TCP grafts established blood flow originating from the endothelium-lined microchannel within a week, and the blood flow covered more areas in the graft over time. In addition, many blood vessels invaded the prevascularized collagen/β-TCP graft and the in vitro preformed microvascular networks anastomosed with the host vasculature, while collagen alone without the support of rigid ceramic scaffold showed less blood vessel invasion and anastomosis. These results suggest a promising strategy for effectively vascularizing large tissue-engineered grafts by integrating multiple hydrogel-based CED-engineered endothelium-lined microchannels into a rigid channeled macroporous scaffold.
Keywords: Collagen; Electrochemical; Microchannel; Vascularization; β-Tricalcium phosphate.
Copyright © 2014 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures


References
-
- Schek RM, Wilke EN, Hollister SJ, Krebsbach PH. Combined use of designed scaffolds and adenoviral gene therapy for skeletal tissue engineering. Biomaterials. 2006;27:1160–6. - PubMed
-
- Jarcho M. Calcium phosphate ceramics as hard tissue prosthetics. Clin Orthop Relat Res. 1981:259–78. - PubMed
-
- LeGeros RZ. Properties of osteoconductive biomaterials: calcium phosphates. Clin Orthop Relat Res. 2002:81–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

