Targeting two-pore domain K(+) channels TREK-1 and TASK-3 for the treatment of depression: a new therapeutic concept
- PMID: 25263033
- PMCID: PMC4301688
- DOI: 10.1111/bph.12953
Targeting two-pore domain K(+) channels TREK-1 and TASK-3 for the treatment of depression: a new therapeutic concept
Abstract
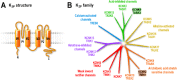
Depression is a disease that is particularly frequent, affecting up to 20% of the population in Western countries. The origins of this pathology involve multiple genes as well as environmental and developmental factors leading to a disorder that remains difficult to treat. Several therapies for depression have been developed and these mainly target monoamine neurotransmitters. However, these treatments are not only associated with numerous adverse effects, but they are also ineffective for more than one-third of patients. Therefore, the need to develop new concepts to treat depression is crucial. Recently, studies using knockout mouse models have provided evidence for a crucial role of two members of the two-pore domain potassium channel (K2P ) family, tandem P-domain weak inward rectifying K(+) (TWIK)-related K(+) channel 1 (TREK-1) and TWIK-related acid-sensitive K(+) channel 3 (TASK-3) in the pathophysiology of depression. It is believed that TREK-1 and TASK-3 antagonists could lead to the development of new antidepressants. Herein, we describe the discovery of spadin, a natural peptide released from the maturation of the neurotensin receptor-3 (also known as sortilin), which specifically blocks the activity of the TREK-1 channel and displays particular antidepressant properties, with a rapid onset of action and the absence of adverse effects. The development of such molecules may open a new era in the field of psychiatry.
© 2014 The British Pharmacological Society.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

