Chaperone-mediated autophagy regulates T cell responses through targeted degradation of negative regulators of T cell activation
- PMID: 25263126
- PMCID: PMC4208273
- DOI: 10.1038/ni.3003
Chaperone-mediated autophagy regulates T cell responses through targeted degradation of negative regulators of T cell activation
Abstract
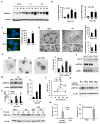
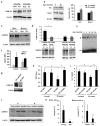
Chaperone-mediated autophagy (CMA) targets soluble proteins for lysosomal degradation. Here we found that CMA was activated in T cells in response to engagement of the T cell antigen receptor (TCR), which induced expression of the CMA-related lysosomal receptor LAMP-2A. In activated T cells, CMA targeted the ubiquitin ligase Itch and the calcineurin inhibitor RCAN1 for degradation to maintain activation-induced responses. Consequently, deletion of the gene encoding LAMP-2A in T cells caused deficient in vivo responses to immunization or infection with Listeria monocytogenes. Impaired CMA activity also occurred in T cells with age, which negatively affected their function. Restoration of LAMP-2A in T cells from old mice resulted in enhancement of activation-induced responses. Our findings define a role for CMA in regulating T cell activation through the targeted degradation of negative regulators of T cell activation.
Figures



References
-
- Dice JF. Peptide sequences that target cytosolic proteins for lysosomal proteolysis. Trends Biochem Sci. 1990;15:305–309. - PubMed
-
- Chiang HL, Terlecky SR, Plant CP, Dice JF. A role for a 70-kilodalton heat shock protein in lysosomal degradation of intracellular proteins. Science. 1989;246:382–385. - PubMed
-
- Cuervo AM, Dice JF. Unique properties of lamp2a compared to other lamp2 isoforms. J Cell Sci. 2000;113 Pt 24:4441–4450. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

