The sweet spot: defining virus-sialic acid interactions
- PMID: 25263223
- PMCID: PMC4791167
- DOI: 10.1038/nrmicro3346
The sweet spot: defining virus-sialic acid interactions
Abstract
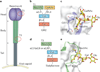
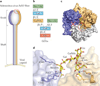
Viral infections are initiated by attachment of the virus to host cell surface receptors, including sialic acid-containing glycans. It is now possible to rapidly identify specific glycan receptors using glycan array screening, to define atomic-level structures of virus-glycan complexes and to alter the glycan-binding site to determine the function of glycan engagement in viral disease. This Review highlights general principles of virus-glycan interactions and provides specific examples of sialic acid binding by viruses with stalk-like attachment proteins, including influenza virus, reovirus, adenovirus and rotavirus. Understanding virus-glycan interactions is essential to combating viral infections and designing improved viral vectors for therapeutic applications.
Figures



References
-
- Barton ES, Connolly JL, Forrest JC, Chappell JD, Dermody TS. Utilization of sialic acid as a coreceptor enhances reovirus attachment by multistep adhesion strengthening. J. Biol. Chem. 2001;276:2200–2211. - PubMed
-
- Rogers GN, et al. Single amino acid substitutions in influenza haemagglutinin change receptor binding specificity. Nature. 1983;304:76–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

