Peri-implant complications for posterior endosteal implants
- PMID: 25263400
- PMCID: PMC4376656
- DOI: 10.1111/clr.12484
Peri-implant complications for posterior endosteal implants
Abstract
Objectives: (1) To assess whether there is evidence of an association between the number of peri-implant tissue complications and patient characteristics such as gender, diabetes status, smoking status, and bite force; (2) To assess whether there is evidence of an association between the number of peri-implant tissue complications and location of the implant, surgical technique used, bone graft status and sinus lift status.
Materials and methods: This randomized, controlled clinical trial included a total of 176 implants (OsseoSpeed, DENTSPLY) in 67 participants with 88 fixed dental prostheses. Information was obtained from health histories, a baseline exam, surgical notes, and post-operative exams. The data were analyzed using Fisher's exact and Mann-Whitney tests and generalized estimating equations using logistic regression with a significance level set at 0.05.
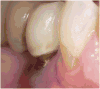
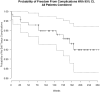
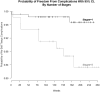
Results: All 176 implants survived within a recall period of 3 years, but 11 implants demonstrated peri-implant tissue complications. Ten sites showed dehiscence and one case exhibited vertical bone loss. There was a statistically significant association between surgical technique used (1-stage or 2-stage) and the presence of soft tissue complications (P = 0.005), where 2-stage surgery was associated with a higher frequency of peri-implant soft tissue complications. A correlation, although not statistically significant (P = 0.077), was noted, between peri-implant tissue complications and bone grafting, suggesting a possible role for this factor as well.
Conclusions: Participants who did not require any second-stage surgery at the implant sites experienced fewer complications. Therefore, additional surgical procedures should be performed judiciously considering their possible effects on peri-implant tissue health.
Clinical significance: The clinical implication of this research study is that secondary surgery should be considered with caution during implant placement and it should be performed only when other options have been exhausted, as it has been shown to have a direct adverse effect on the long-term peri-implant tissue health.
Keywords: bone graft; bone loss; implant failure; implant success; soft tissue complications.
© 2014 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
There are no conflicts of interest associated with this study.
Figures
Similar articles
-
Posterior atrophic jaws rehabilitated with prostheses supported by 5 × 5 mm implants with a nanostructured calcium-incorporated titanium surface or by longer implants in augmented bone. 3-year results from a randomised controlled trial.Eur J Oral Implantol. 2018;11(1):49-61. Eur J Oral Implantol. 2018. PMID: 29557400 Clinical Trial.
-
4 mm long vs longer implants in augmented bone in posterior atrophic jaws: 1-year post-loading results from a multicentre randomised controlled trial.Eur J Oral Implantol. 2018;11(1):31-47. Eur J Oral Implantol. 2018. PMID: 29557399 Clinical Trial.
-
Outcome of implant therapy involving localised lateral alveolar ridge and/or sinus floor augmentation: a clinical and radiographic retrospective 1-year study.Eur J Oral Implantol. 2011 Autumn;4(3):257-67. Eur J Oral Implantol. 2011. PMID: 22043469
-
Implants in the Posterior Maxilla: Open Sinus Lift Versus Conventional Implant Placement. A Systematic Review.Int J Oral Maxillofac Implants. 2019 July/August;34(4):e65–e76. doi: 10.11607/jomi.7274. Epub 2019 Feb 26. Int J Oral Maxillofac Implants. 2019. PMID: 30807622
-
Implant prosthetic rehabilitation in partially edentulous patients with bone atrophy. An umbrella review based on systematic reviews of randomised controlled trials.Eur J Oral Implantol. 2018;11(3):261-280. Eur J Oral Implantol. 2018. PMID: 30246181
Cited by
-
Impact of surgical management in cases of intraoperative membrane perforation during a sinus lift procedure: a follow-up on bone graft stability and implant success.Int J Implant Dent. 2018 Feb 5;4(1):6. doi: 10.1186/s40729-018-0116-8. Int J Implant Dent. 2018. PMID: 29399707 Free PMC article.
-
Biofilms and oral health: nanotechnology for biofilm control.Discov Nano. 2025 Jul 16;20(1):114. doi: 10.1186/s11671-025-04299-3. Discov Nano. 2025. PMID: 40668268 Free PMC article. Review.
-
Perforation of the Schneiderian membrane during sinus floor elevation: a risk factor for long-term success of dental implants?Oral Maxillofac Surg. 2020 Jun;24(2):151-156. doi: 10.1007/s10006-020-00829-8. Epub 2020 Jan 30. Oral Maxillofac Surg. 2020. PMID: 32002693 Free PMC article.
References
-
- Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. The International journal of oral & maxillofacial implants. 1986;1:11–25. - PubMed
-
- Balshi SF, Allen FD, Wolfinger GJ, Balshi TJ. A resonance frequency analysis assessment of maxillary and mandibular immediately loaded implants. The International journal of oral & maxillofacial implants. 2005;20:584–594. - PubMed
-
- Bragger U, Burgin WB, Hammerle CH, Lang NP. Associations between clinical parameters assessed around implants and teeth. Clinical Oral Implants Research. 1997;8:412–421. - PubMed
-
- Buser D, Weber HP, Lang NP. Tissue integration of non-submerged implants. 1-year results of a prospective study with 100 iti hollow-cylinder and hollow-screw implants. Clinical Oral Implants Research. 1990;1:33–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

