Initial testing (stage 1) of the PARP inhibitor BMN 673 by the pediatric preclinical testing program: PALB2 mutation predicts exceptional in vivo response to BMN 673
- PMID: 25263539
- PMCID: PMC4456187
- DOI: 10.1002/pbc.25201
Initial testing (stage 1) of the PARP inhibitor BMN 673 by the pediatric preclinical testing program: PALB2 mutation predicts exceptional in vivo response to BMN 673
Abstract
Background: BMN 673 is a potent inhibitor of poly-ADP ribose polymerase (PARP) that is in clinical testing with a primary focus on BRCA-mutated cancers. BMN 673 is active both through inhibiting PARP catalytic activity and by tightly trapping PARP to DNA at sites of single strand breaks.
Procedure: BMN 673 was tested in vitro at concentrations ranging from 0.1 nM to 1 μM and in vivo at a daily dose of 0.33 mg/kg administered orally twice daily (Mon-Fri) and once daily on weekends (solid tumors) for 28 days.
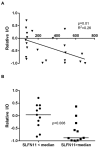
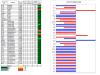
Results: The median relative IC50 (rIC50 ) concentration against the PPTP cell lines was 25.8 nM. The median rIC50 for the Ewing cell lines was lower than for the remaining cell lines (6.4 vs. 31.1 nM, respectively). In vivo BMN 673 induced statistically significant differences in EFS distribution in 17/43 (39.5%) xenograft models. Three objective regressions were observed: a complete response (CR) in a medulloblastoma line (BT-45), a maintained CR in a Wilms tumor line (KT-10), and a maintained CR in an ependymoma line (BT-41). BMN 673 maintained its high level of activity against KT-10 with a threefold reduction in dose. KT-10 possesses a truncating mutation in PALB2 analogous to PALB2 mutations associated with hereditary breast and ovarian cancer that abrogate homologous recombination (HR) repair.
Conclusions: The PPTP results suggest that single agent BMN 673 may have limited clinical activity against pediatric cancers. Single agent activity is more likely for patients whose tumors have defects in HR repair.
Keywords: PALB2; PARP inhibitor; developmental therapeutics; preclinical testing.
© 2014 Wiley Periodicals, Inc.
Conflict of interest statement
Figures

References
-
- Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376(9737):235–244. - PubMed
-
- Fong PC, Yap TA, Boss DS, et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(15):2512–2519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

