Enteroendocrine cells support intestinal stem-cell-mediated homeostasis in Drosophila
- PMID: 25263551
- PMCID: PMC4198943
- DOI: 10.1016/j.celrep.2014.08.052
Enteroendocrine cells support intestinal stem-cell-mediated homeostasis in Drosophila
Abstract
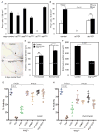
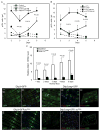
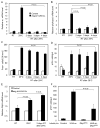
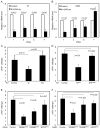
Intestinal stem cells in the adult Drosophila midgut are regulated by growth factors produced from the surrounding niche cells including enterocytes and visceral muscle. The role of the other major cell type, the secretory enteroendocrine cells, in regulating intestinal stem cells remains unclear. We show here that newly eclosed scute loss-of-function mutant flies are completely devoid of enteroendocrine cells. These enteroendocrine cell-less flies have normal ingestion and fecundity but shorter lifespan. Moreover, in these newly eclosed mutant flies, the diet-stimulated midgut growth that depends on the insulin-like peptide 3 expression in the surrounding muscle is defective. The depletion of Tachykinin-producing enteroendocrine cells or knockdown of Tachykinin leads to a similar although less severe phenotype. These results establish that enteroendocrine cells serve as an important link between diet and visceral muscle expression of an insulin-like growth factor to stimulate intestinal stem cell proliferation and tissue growth.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

