Epigenetic regulation of genes that modulate chronic stress-induced visceral pain in the peripheral nervous system
- PMID: 25263804
- PMCID: PMC4274248
- DOI: 10.1053/j.gastro.2014.09.032
Epigenetic regulation of genes that modulate chronic stress-induced visceral pain in the peripheral nervous system
Abstract
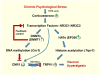
Background & aims: Chronic stress alters the hypothalamic-pituitary-adrenal axis, increases gut motility, and increases the perception of visceral pain. We investigated whether epigenetic mechanisms regulate chronic stress-induced visceral pain in the peripheral nervous systems of rats.
Methods: Male rats were subjected to 1 hour of water avoidance stress each day, or given daily subcutaneous injections of corticosterone, for 10 consecutive days. L4-L5 and L6-S2 dorsal root ganglia (DRG) were collected and compared between stressed and control rats (placed for 1 hour each day in a tank without water). Levels of cannabinoid receptor 1 (CNR1), DNA (cytosine-5-)-methyltransferase 1 (DNMT1), transient receptor potential vanilloid type 1 (TRPV1), and EP300 were knocked down in DRG neurons in situ with small interfering RNAs. We measured DNA methylation and histone acetylation at genes encoding the glucocorticoid receptor (NR3C1), CNR1, and TRPV1. Visceral pain was measured in response to colorectal distention.
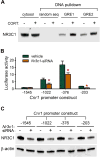
Results: Chronic stress was associated with increased methylation of the Nr3c1 promoter and reduced expression of this gene in L6-S2, but not L4-L5, DRGs. Stress also was associated with up-regulation in DNMT1-associated methylation of the Cnr1 promoter and down-regulation of glucocorticoid-receptor-mediated expression of CNR1 in L6-S2, but not L4-L5, DRGs. Concurrently, chronic stress increased expression of the histone acetyltransferase EP300 and increased histone acetylation at the Trpv1 promoter and expression of the TRPV1 receptor in L6-S2 DRG neurons. Knockdown of DNMT1 and EP300 in L6-S2 DRG neurons of rats reduced DNA methylation and histone acetylation, respectively, and prevented chronic stress-induced increases in visceral pain.
Conclusions: Chronic stress increases DNA methylation and histone acetylation of genes that regulate visceral pain sensation in the peripheral nervous system of rats. Blocking epigenetic regulatory pathways in specific regions of the spinal cord might be developed to treat patients with chronic abdominal pain.
Keywords: Epigenetic Modification; HPA; Pain Sensitivity; Visceral Hyperalgesia.
Copyright © 2015 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
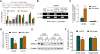



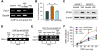
Similar articles
-
Chronic stress and peripheral pain: Evidence for distinct, region-specific changes in visceral and somatosensory pain regulatory pathways.Exp Neurol. 2015 Nov;273:301-11. doi: 10.1016/j.expneurol.2015.09.013. Epub 2015 Sep 25. Exp Neurol. 2015. PMID: 26408049 Free PMC article.
-
Corticosterone mediates reciprocal changes in CB 1 and TRPV1 receptors in primary sensory neurons in the chronically stressed rat.Gastroenterology. 2011 Feb;140(2):627-637.e4. doi: 10.1053/j.gastro.2010.11.003. Epub 2010 Nov 9. Gastroenterology. 2011. PMID: 21070780 Free PMC article.
-
Reciprocal changes in vanilloid (TRPV1) and endocannabinoid (CB1) receptors contribute to visceral hyperalgesia in the water avoidance stressed rat.Gut. 2009 Feb;58(2):202-10. doi: 10.1136/gut.2008.157594. Epub 2008 Oct 20. Gut. 2009. PMID: 18936104 Free PMC article.
-
Stress and glucocorticoid receptor transcriptional programming in time and space: Implications for the brain-gut axis.Neurogastroenterol Motil. 2016 Jan;28(1):12-25. doi: 10.1111/nmo.12706. Neurogastroenterol Motil. 2016. PMID: 26690871 Free PMC article. Review.
-
The Role of the Endocannabinoid System in the Brain-Gut Axis.Gastroenterology. 2016 Aug;151(2):252-66. doi: 10.1053/j.gastro.2016.04.015. Epub 2016 Apr 29. Gastroenterology. 2016. PMID: 27133395 Free PMC article. Review.
Cited by
-
Chronic stress-associated visceral hyperalgesia correlates with severity of intestinal barrier dysfunction.Pain. 2018 Sep;159(9):1777-1789. doi: 10.1097/j.pain.0000000000001271. Pain. 2018. PMID: 29912860 Free PMC article.
-
Towards a systems view of IBS.Nat Rev Gastroenterol Hepatol. 2015 Oct;12(10):592-605. doi: 10.1038/nrgastro.2015.121. Epub 2015 Aug 25. Nat Rev Gastroenterol Hepatol. 2015. PMID: 26303675 Free PMC article. Review.
-
Endocannabinoid Signaling and the Hypothalamic-Pituitary-Adrenal Axis.Compr Physiol. 2016 Dec 6;7(1):1-15. doi: 10.1002/cphy.c160005. Compr Physiol. 2016. PMID: 28134998 Free PMC article. Review.
-
Mechanisms of Stress-induced Visceral Pain.J Neurogastroenterol Motil. 2018 Jan 30;24(1):7-18. doi: 10.5056/jnm17137. J Neurogastroenterol Motil. 2018. PMID: 29291604 Free PMC article. Review.
-
The Transcription Factor Tbx5-Dependent Epigenetic Modification Contributes to Neuropathic Allodynia by Activating TRPV1 Expression in the Dorsal Horn.J Neurosci. 2024 Sep 25;44(39):e0497242024. doi: 10.1523/JNEUROSCI.0497-24.2024. J Neurosci. 2024. PMID: 39174351 Free PMC article.
References
-
- Myer PA, Mannalithara A, Singh G, Singh G, Pasricha PJ, Ladabaum U. Clinical and economic burden of emergency department visits due to gastrointestinal diseases in the United States. Am J Gastroenterol. 2013;108:1496–1507. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

