Precision medicine in chronic disease management: The multiple sclerosis BioScreen
- PMID: 25263997
- PMCID: PMC4214886
- DOI: 10.1002/ana.24282
Precision medicine in chronic disease management: The multiple sclerosis BioScreen
Abstract
We present a precision medicine application developed for multiple sclerosis (MS): the MS BioScreen. This new tool addresses the challenges of dynamic management of a complex chronic disease; the interaction of clinicians and patients with such a tool illustrates the extent to which translational digital medicine-that is, the application of information technology to medicine-has the potential to radically transform medical practice. We introduce 3 key evolutionary phases in displaying data to health care providers, patients, and researchers: visualization (accessing data), contextualization (understanding the data), and actionable interpretation (real-time use of the data to assist decision making). Together, these form the stepping stones that are expected to accelerate standardization of data across platforms, promote evidence-based medicine, support shared decision making, and ultimately lead to improved outcomes.
© 2014 American Neurological Association.
Conflict of interest statement
Dr. Cree has received personal compensation for consulting from Abbvie, Biogen Idec, EMD Serono, Genzyme/Sanofi Aventis, MedImmune, Novartis and Teva Neurosciences and has received contracted research support (including clinical trials) from Acorda, Avanir, Biogen Idec, EMD Serono, Hoffman La Roche and Novartis. Dr. Green serves on Scientific Advisory Board (SAB) for Bionure and for Inception 5; he has served on committees for studies sponsored by Biogen, Medimmune and Novartis, and has served as a consultant for Mylan Pharma, Novartis, Accorda, Prana Pharma and Roche including expert witness work for Mylan. Dr. Gelfand has received compensation for medical legal consulting related to CNS inflammatory disease. Dr. Graves has served as a one-time ad hoc consultant for EMD-Serono.. Dr. Hauser has in the past served on the SAB for BioMarin and Receptos, and currently serves on the SAB of Symbiotix and Bionure. Dr Zamvil has served as a consultant and received honoraria from Biogen-Idec, EMD-Serono, Genzyme, Novartis, Questcor, Roche, and Teva Pharmaceuticals, Inc., and has served or serves on Data Safety Monitoring Boards for Lilly, BioMS, Teva and Opexa Therapeutics.
Figures

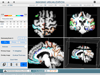




References
-
- Powsner SM, Tufte ER. Graphical summary of patient status. Lancet. 1994;344(8919):386–389. - PubMed
-
- Hood L, Heath JR, Phelps ME, Lin B. Systems biology and new technologies enable predictive and preventative medicine. Science. 2004;306(5696):640–643. - PubMed
-
- Steinhubl SR, Muse ED, Topol EJ. Can Mobile Health Technologies Transform Health Care? JAMA : the journal of the American Medical Association. 2013 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

