Metabolic activation and analgesic effect of flupirtine in healthy subjects, influence of the polymorphic NAT2, UGT1A1 and GSTP1
- PMID: 25264565
- PMCID: PMC4345960
- DOI: 10.1111/bcp.12522
Metabolic activation and analgesic effect of flupirtine in healthy subjects, influence of the polymorphic NAT2, UGT1A1 and GSTP1
Abstract
Aims: The rare association of flupirtine with liver injury is most likely caused by reactive quinone diimines and their oxidative formation may be influenced by the activities of N-acetyltransferases (NAT) that conjugate the less toxic metabolite D13223, and by glucuronosyltransferases (UGT) and glutathione S-transferases (GST) that generate stable terminal glucuronides and mercapturic acid derivatives, respectively. The influence of genetic polymorphisms of NAT2, UGT1A1 and GSTP1 on generation of the terminal mercapturic acid derivatives and analgesic effects was evaluated to identify potential genetic risk factors for hepatotoxicity of flupirtine.
Methods: Metabolic disposition of flupirtine was measured after intravenous administration (100 mg), after swallowing an immediate-release (IR) tablet (100 mg) and after repeated administration of modified release (MR) tablets (400 mg once daily 8 days) in 36 selected healthy subjects. Analgesic effects were measured using pain models (delayed onset of muscle soreness, electric pain).
Results: Flupirtine IR was rapidly but incompletely absorbed (∼ 72%). Repeated administration of flupirtine MR showed lower bioavailability (∼ 60%). Approximately 12% of bioavailable flupirtine IR and 8% of bioavailable flupiritine MR was eliminated as mercapturic acid derivatives into the urine independent of the UGT1A1, NAT2 and GSTP1 genotype. Carriers of variant GSTP1 alleles showed lower bioavailability but increased intestinal secretion of flupirtine and increased efficiency in experimental pain. Flupirtine was not a substrate for ABCB1 and ABCC2.
Conclusions: Formation of mercapturic acid derivatives is a major elimination route for flupirtine in man. However, the theoretically toxic pathway is not influenced by the frequent polymorphisms of UGT1A1, NAT2 and GSTP1.
Keywords: ABCB1; ABCC2; drug-induced liver disease; flupirtine; pharmacogenetics; quinone diimines.
© 2014 The British Pharmacological Society.
Figures
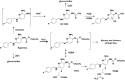

 , flupirtine i.v.;
, flupirtine i.v.;  , flupirtine IR;
, flupirtine IR;  , D13223 i.v.;
, D13223 i.v.;  , D13223 (IR);
, D13223 (IR);  , flupirtine MR;
, flupirtine MR;  , D13223
, D13223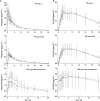
 , GST+;
, GST+;  , GST−
, GST−
References
-
- Kornhuber J, Bleich S, Wiltfang J, Maler M, Parsons CG. Flupirtine shows functional NMDA receptor antagonism by enhancing Mg2+ block via activation of voltage independent potassium channels. Rapid communication. J Neural Transm. 1999;106:857–867. - PubMed
-
- Raffa RB, Pergolizzi JV., Jr The evolving understanding of the analgesic mechanism of action of flupirtine. J Clin Pharm Ther. 2012;37:4–6. - PubMed
-
- Friedel HA, Fitton A. Flupirtine. A review of its pharmacological properties and therapeutic efficacy in pain states. Drugs. 1993;45:548–569. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

