Non-instrumented incubation of a recombinase polymerase amplification assay for the rapid and sensitive detection of proviral HIV-1 DNA
- PMID: 25264766
- PMCID: PMC4180440
- DOI: 10.1371/journal.pone.0108189
Non-instrumented incubation of a recombinase polymerase amplification assay for the rapid and sensitive detection of proviral HIV-1 DNA
Abstract
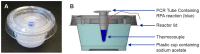
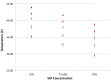
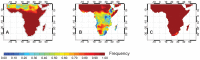
Sensitive diagnostic tests for infectious diseases often employ nucleic acid amplification technologies (NAATs). However, most NAAT assays, including many isothermal amplification methods, require power-dependent instrumentation for incubation. For use in low resource settings (LRS), diagnostics that do not require consistent electricity supply would be ideal. Recombinase polymerase amplification (RPA) is an isothermal amplification technology that has been shown to typically work at temperatures ranging from 25-43°C, and does not require a stringent incubation temperature for optimal performance. Here we evaluate the ability to incubate an HIV-1 RPA assay, intended for use as an infant HIV diagnostic in LRS, at ambient temperatures or with a simple non-instrumented heat source. To determine the range of expected ambient temperatures in settings where an HIV-1 infant diagnostic would be of most use, a dataset of the seasonal range of daily temperatures in sub Saharan Africa was analyzed and revealed ambient temperatures as low as 10°C and rarely above 43°C. All 24 of 24 (100%) HIV-1 RPA reactions amplified when incubated for 20 minutes between 31°C and 43°C. The amplification from the HIV-1 RPA assay under investigation at temperatures was less consistent below 30°C. Thus, we developed a chemical heater to incubate HIV-1 RPA assays when ambient temperatures are between 10°C and 30°C. All 12/12 (100%) reactions amplified with chemical heat incubation from ambient temperatures of 15°C, 20°C, 25°C and 30°C. We also observed that incubation at 30 minutes improved assay performance at lower temperatures where detection was sporadic using 20 minutes incubation. We have demonstrated that incubation of the RPA HIV-1 assay via ambient temperatures or using chemical heaters yields similar results to using electrically powered devices. We propose that this RPA HIV-1 assay may not need dedicated equipment to be a highly sensitive tool to diagnose infant HIV-1 in LRS.
Conflict of interest statement
Figures
Similar articles
-
Rapid detection of HIV-1 proviral DNA for early infant diagnosis using recombinase polymerase amplification.mBio. 2013 Apr 2;4(2):e00135-13. doi: 10.1128/mBio.00135-13. mBio. 2013. PMID: 23549916 Free PMC article.
-
Equipment-free incubation of recombinase polymerase amplification reactions using body heat.PLoS One. 2014 Nov 5;9(11):e112146. doi: 10.1371/journal.pone.0112146. eCollection 2014. PLoS One. 2014. PMID: 25372030 Free PMC article.
-
A wearable microfluidic device for rapid detection of HIV-1 DNA using recombinase polymerase amplification.Talanta. 2019 Dec 1;205:120155. doi: 10.1016/j.talanta.2019.120155. Epub 2019 Jul 17. Talanta. 2019. PMID: 31450450
-
The future in diagnostic tools for TB outbreaks: A review of the approaches with focus on LAMP and RPA diagnostics tests.J Microbiol Methods. 2024 Dec;227:107064. doi: 10.1016/j.mimet.2024.107064. Epub 2024 Oct 22. J Microbiol Methods. 2024. PMID: 39448035 Review.
-
Recombinase polymerase amplification: Emergence as a critical molecular technology for rapid, low-resource diagnostics.Expert Rev Mol Diagn. 2015;15(11):1475-89. doi: 10.1586/14737159.2015.1090877. Epub 2015 Oct 30. Expert Rev Mol Diagn. 2015. PMID: 26517245 Review.
Cited by
-
A Fast, Visual, and Instrument-free Platform Involving Rapid DNA Extraction, Chemical Heating, and Recombinase Aided Amplification for On-Site Nucleic Acid Detection.Front Bioeng Biotechnol. 2021 Nov 22;9:764306. doi: 10.3389/fbioe.2021.764306. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34881235 Free PMC article.
-
A disposable chemical heater and dry enzyme preparation for lysis and extraction of DNA and RNA from microorganisms.Anal Methods. 2016 Apr 14;8(14):2880-2886. doi: 10.1039/c6ay00107f. Epub 2016 Mar 3. Anal Methods. 2016. PMID: 37457919 Free PMC article.
-
A dual RPA-LFD assay for the simultaneous detection of Salmonella typhimurium and Salmonella enteritidis.Front Bioeng Biotechnol. 2024 Mar 8;12:1379939. doi: 10.3389/fbioe.2024.1379939. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38524195 Free PMC article.
-
Development of recombinase polymerase amplification assays for rapid and visual detection of canine distemper virus infecting giant panda.BMC Vet Res. 2021 Apr 23;17(1):172. doi: 10.1186/s12917-021-02880-3. BMC Vet Res. 2021. PMID: 33892731 Free PMC article.
-
Characterization and a RT-RPA assay for rapid detection of Chilli Veinal mottle virus (ChiVMV) in tobacco.Virol J. 2020 Mar 10;17(1):33. doi: 10.1186/s12985-020-01299-w. Virol J. 2020. PMID: 32156292 Free PMC article.
References
-
- World Health Organization (2007) Early detection of HIV infection in infants and children. Guidance note on the selection of technology for the early diagnosis of HIV in infants and children. 1–11.
-
- Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, et al. (2004) Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet 364: 1236–1243. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

