Design and synthesis of C-terminal modified cyclic peptides as VEGFR1 antagonists
- PMID: 25264829
- PMCID: PMC6270838
- DOI: 10.3390/molecules191015391
Design and synthesis of C-terminal modified cyclic peptides as VEGFR1 antagonists
Abstract
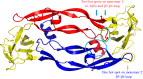
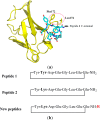
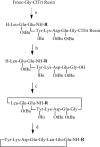
Previously designed cyclic peptide antagonist c[YYDEGLEE]-NH2 disrupts the interaction between vascular endothelial growth factor (VEGF) and its receptors (VEGFRs). It represents a promising tool in the fight against cancer and age-related macular degeneration. We described in this paper the optimization of the lead peptide by C-terminal modification. A new strategy for the synthesis of cyclic peptides is developed, improving the cyclisation efficiency. At 100 µM, several new peptides with an aromatic group flexibly linked at C-terminal end showed significantly increased receptor binding affinities in competition ELISA test. The most active peptide carrying a coumarin group may be a useful tool in anti-angiogenic biological studies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

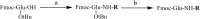
Similar articles
-
Structural studies of the binding of an antagonistic cyclic peptide to the VEGFR1 domain 2.Eur J Med Chem. 2019 May 1;169:65-75. doi: 10.1016/j.ejmech.2019.02.069. Epub 2019 Mar 1. Eur J Med Chem. 2019. PMID: 30856407
-
Biochemical and structural analysis of the binding determinants of a vascular endothelial growth factor receptor peptidic antagonist.J Med Chem. 2010 Jun 10;53(11):4428-40. doi: 10.1021/jm1002167. J Med Chem. 2010. PMID: 20462213
-
Identification of Peptidic Antagonists of Vascular Endothelial Growth Factor Receptor 1 by Scanning the Binding Epitopes of Its Ligands.J Med Chem. 2017 Aug 10;60(15):6598-6606. doi: 10.1021/acs.jmedchem.7b00283. Epub 2017 Jul 21. J Med Chem. 2017. PMID: 28686443
-
Cilengitide: the first anti-angiogenic small molecule drug candidate design, synthesis and clinical evaluation.Anticancer Agents Med Chem. 2010 Dec;10(10):753-68. doi: 10.2174/187152010794728639. Anticancer Agents Med Chem. 2010. PMID: 21269250 Free PMC article. Review.
-
Recent developments in the inhibition of angiogenesis: examples from studies on platelet factor-4 and the VEGF/VEGFR system.Biochem Pharmacol. 2004 Sep 15;68(6):1017-21. doi: 10.1016/j.bcp.2004.05.030. Biochem Pharmacol. 2004. PMID: 15313395 Review.
Cited by
-
Anti-metastatic effect of the TM4SF5-specific peptide vaccine and humanized monoclonal antibody on colon cancer in a mouse lung metastasis model.Oncotarget. 2016 Nov 29;7(48):79170-79186. doi: 10.18632/oncotarget.13005. Oncotarget. 2016. PMID: 27816969 Free PMC article.
-
Cyclic Peptides for the Treatment of Cancers: A Review.Molecules. 2022 Jul 11;27(14):4428. doi: 10.3390/molecules27144428. Molecules. 2022. PMID: 35889301 Free PMC article. Review.
-
Considering both small and large scale motions of vascular endothelial growth factor (VEGF) is crucial for reliably predicting its binding affinities to DNA aptamers.RSC Adv. 2021 Mar 1;11(16):9315-9326. doi: 10.1039/d0ra10106k. eCollection 2021 Mar 1. RSC Adv. 2021. PMID: 35423456 Free PMC article.
-
The Research Progress of Bioactive Peptides Derived from Traditional Natural Products in China.Molecules. 2023 Sep 3;28(17):6421. doi: 10.3390/molecules28176421. Molecules. 2023. PMID: 37687249 Free PMC article. Review.
-
Analyzing VEGFA/VEGFR1 Interaction: Application of the Resonant Recognition Model-Stockwell Transform Method to Explore Potential Therapeutics for Angiogenesis-Related Diseases.Protein J. 2024 Aug;43(4):697-710. doi: 10.1007/s10930-024-10219-8. Epub 2024 Jul 16. Protein J. 2024. PMID: 39014261
References
-
- Carmeliet P. Angiogenesis in health and disease. Nat. Med. 2003;9:653–660. - PubMed
-
- Kowanetz M., Ferrara N. Vascular endothelial growth factor signaling pathways: Therapeutic perspective. Clin. Cancer Res. 2006;17:5018–5022. - PubMed
-
- Ferrara N., Gerber H.P., LeCouter J. The biology of VEGF and its receptors. Nat. Med. 2003;9:669–676. - PubMed
-
- Ferrara N., Kerbel R.S. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

