N-acetylcysteine attenuates ischemia-reperfusion-induced apoptosis and autophagy in mouse liver via regulation of the ROS/JNK/Bcl-2 pathway
- PMID: 25264893
- PMCID: PMC4181678
- DOI: 10.1371/journal.pone.0108855
N-acetylcysteine attenuates ischemia-reperfusion-induced apoptosis and autophagy in mouse liver via regulation of the ROS/JNK/Bcl-2 pathway
Abstract
Background: Hepatic ischemia-reperfusion injury (HIRI) remains a pivotal clinical problem after hemorrhagic shock, transplantation, and some types of toxic hepatic injury. Apoptosis and autophagy play important roles in cell death during HIRI. It is also known that N-acetylcysteine (NAC) has significant pharmacologic effects on HIRI including elimination of reactive oxygen species (ROS) and attenuation of hepatic apoptosis. However, the effects of NAC on HIRI-induced autophagy have not been reported. In this study, we evaluated the effects of NAC on autophagy and apoptosis in HIRI, and explored the possible mechanism involved.
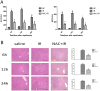
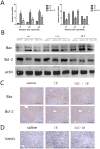
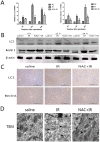
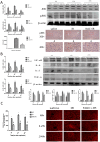
Methods: A mouse model of segmental (70%) hepatic warm ischemia was adopted to determine hepatic injury. NAC (150 mg/kg), a hepatoprotection agent, was administered before surgery. We hypothesized that the mechanism of NAC may involve the ROS/JNK/Bcl-2 pathway. We evaluated the expression of JNK, P-JNK, Bcl-2, Beclin 1 and LC3 by western blotting and immunohistochemical staining. Autophagosomes were evaluated by transmission electron microscopy (TEM).
Results: We found that ALT, AST and pathological changes were significantly improved in the NAC group. Western blotting analysis showed that the expression levels of Beclin 1 and LC3 were significantly decreased in NAC-treated mice. In addition, JNK, p-JNK, Bax, TNF-α, NF-κB, IL2, IL6 and levels were also decreased in NAC-treated mice.
Conclusion: NAC can prevent HIRI-induced autophagy and apoptosis by influencing the JNK signal pathway. The mechanism is likely to involve attenuation of JNK and p-JNK via scavenged ROS, an indirect increase in Bcl-2 level, and finally an alteration in the balance of Beclin 1 and Bcl-2.
Conflict of interest statement
Figures
Similar articles
-
15-Deoxy-Δ12,14-prostaglandin J2 alleviates hepatic ischemia-reperfusion injury in mice via inducing antioxidant response and inhibiting apoptosis and autophagy.Acta Pharmacol Sin. 2017 May;38(5):672-687. doi: 10.1038/aps.2016.108. Epub 2017 Feb 20. Acta Pharmacol Sin. 2017. PMID: 28216619 Free PMC article.
-
N-acetylcysteine attenuates reactive-oxygen-species-mediated endoplasmic reticulum stress during liver ischemia-reperfusion injury.World J Gastroenterol. 2014 Nov 7;20(41):15289-98. doi: 10.3748/wjg.v20.i41.15289. World J Gastroenterol. 2014. PMID: 25386077 Free PMC article.
-
The protective effects of shikonin on hepatic ischemia/reperfusion injury are mediated by the activation of the PI3K/Akt pathway.Sci Rep. 2017 Mar 21;7:44785. doi: 10.1038/srep44785. Sci Rep. 2017. PMID: 28322249 Free PMC article.
-
Targeting NF-κB in Hepatic Ischemia-Reperfusion Alleviation: from Signaling Networks to Therapeutic Targeting.Mol Neurobiol. 2024 Jun;61(6):3409-3426. doi: 10.1007/s12035-023-03787-w. Epub 2023 Nov 22. Mol Neurobiol. 2024. PMID: 37991700 Review.
-
Multimodal modulation of hepatic ischemia/reperfusion-induced injury by phytochemical agents: A mechanistic evaluation of hepatoprotective potential and safety profiles.Int Immunopharmacol. 2024 Sep 10;138:112445. doi: 10.1016/j.intimp.2024.112445. Epub 2024 Jun 29. Int Immunopharmacol. 2024. PMID: 38944946 Review.
Cited by
-
Modification of Preservative Fluids with Antioxidants in Terms of Their Efficacy in Liver Protection before Transplantation.Int J Mol Sci. 2024 Feb 3;25(3):1850. doi: 10.3390/ijms25031850. Int J Mol Sci. 2024. PMID: 38339128 Free PMC article. Review.
-
Sex Differences in X-ray-Induced Endothelial Damage: Effect of Taurine and N-Acetylcysteine.Antioxidants (Basel). 2022 Dec 29;12(1):77. doi: 10.3390/antiox12010077. Antioxidants (Basel). 2022. PMID: 36670939 Free PMC article.
-
N-Acetylcysteine Prevents Post-embolization Syndrome in Patients with Hepatocellular Carcinoma Following Transarterial Chemoembolization.Dig Dis Sci. 2019 Nov;64(11):3337-3345. doi: 10.1007/s10620-019-05652-0. Epub 2019 May 9. Dig Dis Sci. 2019. PMID: 31073737 Clinical Trial.
-
Hypoxia Potentiates Palmitate-induced Pro-inflammatory Activation of Primary Human Macrophages.J Biol Chem. 2016 Jan 1;291(1):413-24. doi: 10.1074/jbc.M115.686709. Epub 2015 Nov 17. J Biol Chem. 2016. PMID: 26578520 Free PMC article.
-
Oxidative Stress and Inflammation in Hepatic Diseases: Therapeutic Possibilities of N-Acetylcysteine.Int J Mol Sci. 2015 Dec 18;16(12):30269-308. doi: 10.3390/ijms161226225. Int J Mol Sci. 2015. PMID: 26694382 Free PMC article. Review.
References
-
- Toledo-Pereyra LH, Simmons RL, Najarian JS (1975) Protection of the ischemic liver by donor pretreatment before transplantation. Am J Surg 129: 513–517. - PubMed
-
- Teoh NC, Farrell GC (2003) Hepatic ischemia reperfusion injury: pathogenic mechanisms and basis for hepatoprotection. J Gastroenterol Hepatol 18: 891–902. - PubMed
-
- Teoh NC (2011) Hepatic ischemia reperfusion injury: Contemporary perspectives on pathogenic mechanisms and basis for hepatoprotection-the good, bad and deadly. J Gastroenterol Hepatol 26 Suppl 1: 180–187. - PubMed
-
- Jaeschke H, Lemasters JJ (2003) Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology 125: 1246–1257. - PubMed
-
- Theodoraki K, Tympa A, Karmaniolou I, Tsaroucha A, Arkadopoulos N, et al. (2011) Ischemia/reperfusion injury in liver resection: a review of preconditioning methods. Surg Today 41: 620–629. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

