Distinct conformational behaviors of four mammalian dual-flavin reductases (cytochrome P450 reductase, methionine synthase reductase, neuronal nitric oxide synthase, endothelial nitric oxide synthase) determine their unique catalytic profiles
- PMID: 25265015
- PMCID: PMC4245374
- DOI: 10.1111/febs.13073
Distinct conformational behaviors of four mammalian dual-flavin reductases (cytochrome P450 reductase, methionine synthase reductase, neuronal nitric oxide synthase, endothelial nitric oxide synthase) determine their unique catalytic profiles
Abstract
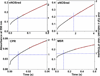
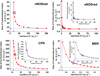
Multidomain enzymes often rely on large conformational motions to function. However, the conformational setpoints, rates of domain motions and relationships between these parameters and catalytic activity are not well understood. To address this, we determined and compared the conformational setpoints and the rates of conformational switching between closed unreactive and open reactive states in four mammalian diflavin NADPH oxidoreductases that catalyze important biological electron transfer reactions: cytochrome P450 reductase, methionine synthase reductase and endothelial and neuronal nitric oxide synthase. We used stopped-flow spectroscopy, single turnover methods and a kinetic model that relates electron flux through each enzyme to its conformational setpoint and its rates of conformational switching. The results show that the four flavoproteins, when fully-reduced, have a broad range of conformational setpoints (from 12% to 72% open state) and also vary 100-fold with respect to their rates of conformational switching between unreactive closed and reactive open states (cytochrome P450 reductase > neuronal nitric oxide synthase > methionine synthase reductase > endothelial nitric oxide synthase). Furthermore, simulations of the kinetic model could explain how each flavoprotein can support its given rate of electron flux (cytochrome c reductase activity) based on its unique conformational setpoint and switching rates. The present study is the first to quantify these conformational parameters among the diflavin enzymes and suggests how the parameters might be manipulated to speed or slow biological electron flux.
Keywords: conformational equilibrium; electron transfer; flavoprotein; kinetic model; nitric oxide.
© 2014 FEBS.
Figures
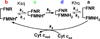





References
-
- De CL, Mattevi A. New frontiers in structural flavoenzymology. Curr. Opin. Struct. Biol. 2006;16:722–728. - PubMed
-
- Iyanagi T. Structure and function of NADPH-cytochrome P450 reductase and nitric oxide synthase reductase domain. Biochem. Biophys. Res. Commun. 2005;338:520–528. - PubMed
-
- Joosten V, van Berkel WJ. Flavoenzymes. Curr. Opin. Chem. Biol. 2007;11:195–202. - PubMed
-
- Gomez-Moreno C. New roles of flavoproteins in molecular cell biology. FEBS J. 2009;276:4289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

