Prohibitin-mediated lifespan and mitochondrial stress implicate SGK-1, insulin/IGF and mTORC2 in C. elegans
- PMID: 25265021
- PMCID: PMC4180437
- DOI: 10.1371/journal.pone.0107671
Prohibitin-mediated lifespan and mitochondrial stress implicate SGK-1, insulin/IGF and mTORC2 in C. elegans
Abstract
Lifespan regulation by mitochondrial proteins has been well described, however, the mechanism of this regulation is not fully understood. Amongst the mitochondrial proteins profoundly affecting ageing are prohibitins (PHB-1 and PHB-2). Paradoxically, in C. elegans prohibitin depletion shortens the lifespan of wild type animals while dramatically extending that of metabolically compromised animals, such as daf-2-insulin-receptor mutants. Here we show that amongst the three kinases known to act downstream of daf-2, only loss of function of sgk-1 recapitulates the ageing phenotype observed in daf-2 mutants upon prohibitin depletion. Interestingly, signalling through SGK-1 receives input from an additional pathway, parallel to DAF-2, for the prohibitin-mediated lifespan phenotype. We investigated the effect of prohibitin depletion on the mitochondrial unfolded protein response (UPRmt). Remarkably, the lifespan extension upon prohibitin elimination, of both daf-2 and sgk-1 mutants, is accompanied by suppression of the UPRmt induced by lack of prohibitin. On the contrary, gain of function of SGK-1 results in further shortening of lifespan and a further increase of the UPRmt in prohibitin depleted animals. Moreover, SGK-1 interacts with RICT-1 for the regulation of the UPRmt in a parallel pathway to DAF-2. Interestingly, prohibitin depletion in rict-1 loss of function mutant animals also causes lifespan extension. Finally, we reveal an unprecedented role for mTORC2-SGK-1 in the regulation of mitochodrial homeostasis. Together, these results give further insight into the mechanism of lifespan regulation by mitochondrial function and reveal a cross-talk of mitochondria with two key pathways, Insulin/IGF and mTORC2, for the regulation of ageing and stress response.
Conflict of interest statement
Figures
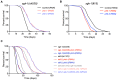

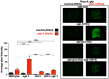
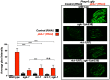




References
-
- Coates PJ, Jamieson DJ, Smart K, Prescott AR, Hall PA (1997) The prohibitin family of mitochondrial proteins regulate replicative lifespan. Curr Biol 7: 607–610. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

