Nutritional advice in older patients at risk of malnutrition during treatment for chemotherapy: a two-year randomized controlled trial
- PMID: 25265392
- PMCID: PMC4181649
- DOI: 10.1371/journal.pone.0108687
Nutritional advice in older patients at risk of malnutrition during treatment for chemotherapy: a two-year randomized controlled trial
Abstract
Objective: We tested the effect of dietary advice dedicated to increase intake in older patients at risk for malnutrition during chemotherapy, versus usual care, on one-year mortality.
Method: We conducted a multicentre, open-label interventional, stratified (centre), parallel randomised controlled trial, with a 1∶1 ratio, with two-year follow-up. Patients were aged 70 years or older treated with chemotherapy for solid tumour and at risk of malnutrition (MNA, Mini Nutritional Assessment 17-23.5). Intervention consisted of diet counselling with the aim of achieving an energy intake of 30 kCal/kg body weight/d and 1.2 g protein/kg/d, by face-to-face discussion targeting the main nutritional symptoms, compared to usual care. Interviews were performed 6 times during the chemotherapy sessions for 3 to 6 months. The primary endpoint was 1-year mortality and secondary endpoints were 2-year mortality, toxicities and chemotherapy outcomes.
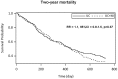
Results: Between April 2007 and March 2010 we randomised 341 patients and 336 were analysed: mean (standard deviation) age of 78.0 y (4·9), 51.2% male, mean MNA 20.2 (2.1). Distribution of cancer types was similar in the two groups; the most frequent were colon (22.4%), lymphoma (14.9%), lung (10.4%), and pancreas (17.0%). Both groups increased their dietary intake, but to a larger extent with intervention (p<0.01). At the second visit, the energy target was achieved in 57 (40.4%) patients and the protein target in 66 (46.8%) with the intervention compared respectively to 13 (13.5%) and 20 (20.8%) in the controls. Death occurred during the first year in 143 patients (42.56%), without difference according to the intervention (p = 0.79). No difference in nutritional status changes was found. Response to chemotherapy was also similar between the groups.
Conclusion: Early dietary counselling was efficient in increasing intake but had no beneficial effect on mortality or secondary outcomes. Cancer cachexia antianabolism may explain this lack of effect.
Trial registration: ClinicalTrials.gov NCT00459589.
Conflict of interest statement
Figures

References
-
- Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, et al. (1980) Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med 69: 491–497. - PubMed
-
- Arends J, Bodoky G, Bozzetti F, Fearon K, Muscaritoli M, et al. (2006) ESPEN Guidelines on Enteral Nutrition: Non-surgical oncology. Clin Nutr 25: 245–259. - PubMed
-
- Senesse P, Bachmann P, Bensadoun RJ, Besnard I, Bourdel-Marchasson I, et al. (2014) Clinical nutrition guidelines of the French Speaking Society of Clinical Nutrition and Metabolism (SFNEP): Summary of recommendations for adults undergoing non-surgical anticancer treatment. Dig Liver Dis 46: 867–874. - PubMed
-
- Senesse P, Assenat E, Schneider S, Chargari C, Magne N, et al. (2008) Nutritional support during oncologic treatment of patients with gastrointestinal cancer: who could benefit? Cancer Treat Rev 34: 568–575. - PubMed
-
- Baldwin C, Spiro A, McGough C, Norman AR, Gillbanks A, et al. (2011) Simple nutritional intervention in patients with advanced cancers of the gastrointestinal tract, non-small cell lung cancers or mesothelioma and weight loss receiving chemotherapy: a randomised controlled trial. J Hum Nutr Diet 24: 431–440. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

