Clinical efficacy and safety of pamidronate therapy on bone mass density in early post-renal transplant period: a meta-analysis of randomized controlled trials
- PMID: 25265508
- PMCID: PMC4180498
- DOI: 10.1371/journal.pone.0108106
Clinical efficacy and safety of pamidronate therapy on bone mass density in early post-renal transplant period: a meta-analysis of randomized controlled trials
Abstract
Introduction: The overall effect of pamidronate on bone mass density (BMD) in the early renal transplant period varies considerably among studies. The effects of pamidronate on graft function have not been determined.
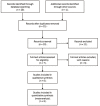
Materials and methods: A comprehensive search was conducted in PubMed, the Cochrane Central Register of Controlled Trials (CENTRAL) and Embase independently by two authors. Randomized controlled trials of pamidronate evaluating bone loss in the first year of renal transplantation were included. Methods reported in the "Cochrane Handbook for Systematic Reviews of Interventions 5.0.2" were used to evaluate changes of lumbar spine and femoral neck BMD, and serum creatinine, calcium and intact parathyroid hormone (iPTH) levels. Fixed or random effect models were used as appropriate.
Results: Six randomized trials evaluating 281 patients were identified. One hundred forty-four were treated with pamidronate and 137 were control patients. Administration of pamidronate was associated with significant reduction of bone loss in the lumbar spine, compared to the control group (standardized mean difference (SMD) = 24.62 [16.25, 32.99]). There was no difference between the pamidronate treated and control femoral neck BMD (SMD = 3.53 [-1.84, 8.90]). A significant increase in the serum creatinine level of the intervention group was seen, compared to the control group. The serum calcium and iPTH of the pamidronate and control groups were not different after 1 year (serum creatinine: SMD = -3.101 [-5.33, -0.89]; serum calcium: SMD = 2.18 [-0.8, 5.16]; serum iPTH: SMD = 0.06 [-0.19, 0.31]). Heterogeneity was low for serum calcium and iPTH and high for serum creatinine.
Conclusions: This meta-analysis demonstrated the beneficial clinical efficacy of pamidronate on BMD with no association with any alteration in graft function during the first year of renal transplantation. Significant heterogeneity precludes the conclusion of the relationship between serum creatinine and pamidronate.
Conflict of interest statement
Figures


References
-
- Epstein S, Stuss M (2011) Transplantation osteoporosis. Endokrynol Pol 62: 472–485. - PubMed
-
- Okamoto M, Suzuki T, Fujiki M, Nobori S, Ushigome H, et al. (2010) The consequences for live kidney donors with preexisting glucose intolerance without diabetic complication: analysis at a single Japanese center. Transplantation 89: 1391–1395. - PubMed
-
- Smerud KT, Dolgos S, Olsen IC, Asberg A, Sagedal S, et al. (2012) A 1-year randomized, double-blind, placebo-controlled study of intravenous ibandronate on bone loss following renal transplantation. Am J Transplant 12: 3316–3325. - PubMed
-
- Papapoulos SE (2008) Bisphosphonates: how do they work? Best Pract Res Clin Endocrinol Metab 22: 831–847. - PubMed
-
- Mitterbauer C, Schwarz C, Haas M, Oberbauer R (2006) Effects of bisphosphonates on bone loss in the first year after renal transplantation–a meta-analysis of randomized controlled trials. Nephrol Dial Transplant 21: 2275–2281. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

