The development of immunoconjugates for targeted cancer therapy
- PMID: 25265912
- PMCID: PMC4700536
- DOI: 10.1038/nrclinonc.2014.159
The development of immunoconjugates for targeted cancer therapy
Abstract
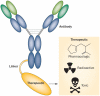
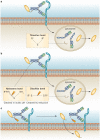
Immunoconjugates are specific, highly effective, minimally toxic anticancer therapies that are beginning to show promise in the clinic. Immunoconjugates consist of three separate components: an antibody that binds to a cancer cell antigen with high specificity, an effector molecule that has a high capacity to kill the cancer cell, and a linker that will ensure the effector does not separate from the antibody during transit and will reliably release the effector to the cancer cell or tumour stroma. The high affinity antibody-antigen interaction allows specific and selective delivery of a range of effectors, including pharmacologic agents, radioisotopes, and toxins, to cancer cells. Some anticancer molecules are not well tolerated when administered systemically owing to unacceptable toxicity to the host. However, this limitation can be overcome through the linking of such cytotoxins to specific antibodies, which mask the toxic effects of the drug until it reaches its target. Conversely, many unconjugated antibodies are highly specific for a cancer target, but have low therapeutic potential and can be repurposed as delivery vehicles for highly potent effectors. In this Review, we summarize the successes and shortcomings of immunoconjugates, and discuss the future potential for the development of these therapies.
Figures

Comment in
-
CD30-targeting immunoconjugates and bystander effects.Nat Rev Clin Oncol. 2015 Apr;12(4). doi: 10.1038/nrclinonc.2014.159-c1. Epub 2015 Mar 3. Nat Rev Clin Oncol. 2015. PMID: 25734636 No abstract available.
References
-
- Paul Ehrlich—Biographical. Nobelprize.org. 2014 [online], http://www.nobelprize.org/nobel_prizes/medicine/laureates/1908/ehrlich-b....
-
- Anderson CS, Quinones R, Olson MR. Determining efficacy and side effects from the concurrent use of chemotherapy and radiation therapy for the management of adult solid tumors [abstract] J. Clin. Oncol. 2014;32(Suppl.):e17640.
-
- Van Cutsem E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N. Engl. J. Med. 2009;360:1408–1417. - PubMed
-
- Fuchs CS, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C study. J. Clin. Oncol. 2007;25:4779–4786. - PubMed
-
- Hochster HS, et al. Safety and efficacy of oxaliplatin-fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer (mCRC): results of the TREE-Study. J. Clin. Oncol. 2008;26:3523–3529. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

