Development of the National Cancer Institute's patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE)
- PMID: 25265940
- PMCID: PMC4200059
- DOI: 10.1093/jnci/dju244
Development of the National Cancer Institute's patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE)
Abstract
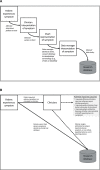
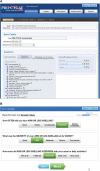
The standard approach for documenting symptomatic adverse events (AEs) in cancer clinical trials involves investigator reporting using the National Cancer Institute's (NCI's) Common Terminology Criteria for Adverse Events (CTCAE). Because this approach underdetects symptomatic AEs, the NCI issued two contracts to create a patient-reported outcome (PRO) measurement system as a companion to the CTCAE, called the PRO-CTCAE. This Commentary describes development of the PRO-CTCAE by a group of multidisciplinary investigators and patient representatives and provides an overview of qualitative and quantitative studies of its measurement properties. A systematic evaluation of all 790 AEs listed in the CTCAE identified 78 appropriate for patient self-reporting. For each of these, a PRO-CTCAE plain language term in English and one to three items characterizing the frequency, severity, and/or activity interference of the AE were created, rendering a library of 124 PRO-CTCAE items. These items were refined in a cognitive interviewing study among patients on active cancer treatment with diverse educational, racial, and geographic backgrounds. Favorable measurement properties of the items, including construct validity, reliability, responsiveness, and between-mode equivalence, were determined prospectively in a demographically diverse population of patients receiving treatments for many different tumor types. A software platform was built to administer PRO-CTCAE items to clinical trial participants via the internet or telephone interactive voice response and was refined through usability testing. Work is ongoing to translate the PRO-CTCAE into multiple languages and to determine the optimal approach for integrating the PRO-CTCAE into clinical trial workflow and AE analyses. It is envisioned that the PRO-CTCAE will enhance the precision and patient-centeredness of adverse event reporting in cancer clinical research.
© The Author 2014. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Trotti A, Colevas AD, Setser A, Basch E. Patient-Reported Outcomes and the Evolution of Adverse Event Reporting in Oncology. J Clin Oncol. 2007;25(32):5121–5127 - PubMed
-
- Fromme EK, Eilers KM, Mori M, Hsieh YC, Beer TM. How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient-reported symptoms from the Quality-of-Life Questionnaire C30. J Clin Oncol. 2004;22(17):3485–3490 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

