Retinoic acid negatively regulates dact3b expression in the hindbrain of zebrafish embryos
- PMID: 25266145
- PMCID: PMC4254272
- DOI: 10.1016/j.gep.2014.09.003
Retinoic acid negatively regulates dact3b expression in the hindbrain of zebrafish embryos
Abstract
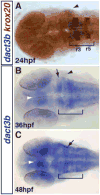
Wnt signaling plays important roles in normal development as well as pathophysiological conditions. The Dapper antagonist of β-catenin (Dact) proteins are modulators of both canonical and non-canonical Wnt signaling via direct interactions with Dishevelled (Dvl) and Van Gogh like-2 (Vangl2). Here, we report the dynamic expression patterns of two zebrafish dact3 paralogs during early embryonic development. Our whole mount in situ hybridization (WISH) analysis indicates that specific dact3a expression starts by the tailbud stage in adaxial cells. Later, it is expressed in the anterior lateral plate mesoderm, somites, migrating cranial neural crest, and hindbrain neurons. By comparison, dact3b expression initiates on the dorsal side at the dome stage and soon after is expressed in the dorsal forerunner cells (DFCs) during gastrulation. At later stages, dact3b expression becomes restricted to the branchial neurons of the hindbrain and to the second pharyngeal arch. To investigate how zebrafish dact3 gene expression is regulated, we manipulated retinoic acid (RA) signaling during development and found that it negatively regulates dact3b in the hindbrain. Our study is the first to document the expression of the paralogous zebrafish dact3 genes during early development and demonstrate dact3b can be regulated by RA signaling. Therefore, our study opens up new avenues to study Dact3 function in the development of multiple tissues and suggests a previously unappreciated cross regulation of Wnt signaling by RA signaling in the developing vertebrate hindbrain.
Keywords: Dact; Dishevelled; Retinoic acid signaling; Vertebrate development; Wnt signaling.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures






References
-
- Ahn D, Ho RK. Tri-phasic expression of posterior Hox genes during development of pectoral fins in zebrafish: implications for the evolution of vertebrate paired appendages. Developmental biology. 2008;322:220–233. - PubMed
-
- Alvares L, Winterbottom F, Jorge E, Rodrigues Sobreira D, Xavier-Neto J, Schubert F, Dietrich S. Chicken dapper genes are versatile markers for mesodermal tissues, embryonic muscle stem cells, neural crest cells, and neurogenic placodes. Developmental dynamics: an official publication of the American Association of Anatomists. 2009;238:1166–1178. - PubMed
-
- Astolfi A, Nannini M, Pantaleo M, Di Battista M, Heinrich M, Santini D, Catena F, Corless C, Maleddu A, Saponara M, Lolli C, Di Scioscio V, Formica S, Biasco G. A molecular portrait of gastrointestinal stromal tumors: an integrative analysis of gene expression profiling and high-resolution genomic copy number. Laboratory investigation; a journal of technical methods and pathology. 2010;90:1285–1294. - PubMed
-
- Auclair F, Vald√©s N, Marchand R. Rhombomere-specific origin of branchial and visceral motoneurons of the facial nerve in the rat embryo. The Journal of comparative neurology. 1996;369:451–461. - PubMed
-
- Brott B, Sokol S. Frodo proteins: modulators of Wnt signaling in vertebrate development. Differentiation; research in biological diversity. 2005;73:323–329. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

