Dendritic cells and macrophages in the kidney: a spectrum of good and evil
- PMID: 25266210
- PMCID: PMC4922410
- DOI: 10.1038/nrneph.2014.170
Dendritic cells and macrophages in the kidney: a spectrum of good and evil
Abstract
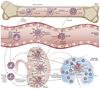
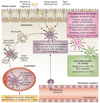
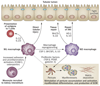
Renal dendritic cells (DCs) and macrophages represent a constitutive, extensive and contiguous network of innate immune cells that provide sentinel and immune-intelligence activity; they induce and regulate inflammatory responses to freely filtered antigenic material and protect the kidney from infection. Tissue-resident or infiltrating DCs and macrophages are key factors in the initiation and propagation of renal disease, as well as essential contributors to subsequent tissue regeneration, regardless of the aetiological and pathogenetic mechanisms. The identification, and functional and phenotypic distinction of these cell types is complex and incompletely understood, and the same is true of their interplay and relationships with effector and regulatory cells of the adaptive immune system. In this Review, we discuss the common and distinct characteristics of DCs and macrophages, as well as key advances that have identified the renal-specific functions of these important phagocytic, antigen-presenting cells, and their roles in potentiating or mitigating intrinsic kidney disease. We also identify remaining issues that are of priority for further investigation, and highlight the prospects for translational and therapeutic application of the knowledge acquired.
Conflict of interest statement
Conflict of Interest: JSI is chair of the Scientific Advisory Boards of Vasculox (St Louis, MO) and Radiation Control Technologies (Rockville, MD). AWT is co-inventor of a US patent for the generation of tolerogenic dendritic cells to promote transplant tolerance.
Figures

References
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. - PubMed
-
- Geissmann F. The origin of dendritic cells. Nat Immunol. 2007;8:558–60. - PubMed
-
- Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–61. - PubMed
-
- Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7:19–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

