Graft versus leukemia response without graft-versus-host disease elicited by adoptively transferred multivirus-specific T-cells
- PMID: 25266309
- PMCID: PMC4426803
- DOI: 10.1038/mt.2014.192
Graft versus leukemia response without graft-versus-host disease elicited by adoptively transferred multivirus-specific T-cells
Abstract
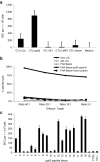
A 12-year-old boy with refractory acute lymphoblastic leukemia received a haploidentical transplant from his mother. As prophylaxis for Epstein-Barr virus (EBV), cytomegalovirus (CMV) and adenovirus, he received ex vivo expanded virus-specific donor T cells 3.5 months after transplant. Four weeks later leukemic blasts bearing the E2A deletion, identified by fluorescent in situ hybridization (FISH), appeared transiently in the blood followed by a FISH-negative hematological remission, which was sustained until a testicular relapse 3.5 months later. Clearance of the circulating leukemic cells coincided with a marked increase in circulating virus-specific T cells. The virus-specific cytotoxic T-cell (CTL) line showed strong polyfunctional reactivity with the patient's leukemic cells but not phytohemagglutinin (PHA) blasts, suggesting that virus-specific CTL lines may have clinically significant antileukemia activity.
Figures


References
-
- Ribera JM, Ortega JJ, Oriol A, Bastida P, Calvo C, Pérez-Hurtado JM, et al. Comparison of intensive chemotherapy, allogeneic, or autologous stem-cell transplantation as postremission treatment for children with very high risk acute lymphoblastic leukemia: PETHEMA ALL-93 Trial. J Clin Oncol. 2007;25:16–24. - PubMed
-
- Saarinen-Pihkala UM, Heilmann C, Winiarski J, Glomstein A, Abrahamsson J, Arvidson J, et al. Pathways through relapses and deaths of children with acute lymphoblastic leukemia: role of allogeneic stem-cell transplantation in Nordic data. J Clin Oncol. 2006;24:5750–5762. - PubMed
-
- Weiden PL, Flournoy N, Thomas ED, Prentice R, Fefer A, Buckner CD, et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med. 1979;300:1068–1073. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

