Characterization of p-hydroxycinnamate catabolism in a soil Actinobacterium
- PMID: 25266382
- PMCID: PMC4248857
- DOI: 10.1128/JB.02247-14
Characterization of p-hydroxycinnamate catabolism in a soil Actinobacterium
Abstract
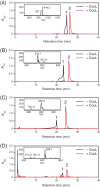
p-Hydroxycinnamates, such as ferulate and p-coumarate, are components of plant cell walls and have a number of commercial applications. Rhodococcus jostii RHA1 (RHA1) catabolizes ferulate via vanillate and the β-ketoadipate pathway. Here, we used transcriptomics to identify genes in RHA1 that are upregulated during growth on ferulate versus benzoate. The upregulated genes included three transcriptional units predicted to encode the uptake and β-oxidative deacetylation of p-hydroxycinnamates: couHTL, couNOM, and couR. Neither ΔcouL mutants nor ΔcouO mutants grew on p-hydroxycinnamates, but they did grow on vanillate. Among several p-hydroxycinnamates, CouL catalyzed the thioesterification of p-coumarate and caffeate most efficiently (k(cat)/K(m) = ∼ 400 mM(-1) s(-1)). p-Coumarate was also RHA1's preferred growth substrate, suggesting that CouL is a determinant of the pathway's specificity. CouL did not catalyze the activation of sinapate, in similarity to two p-coumaric acid:coenzyme A (CoA) ligases from plants, and contains the same bulged loop that helps determine substrate specificity in the plant homologues. The couO mutant accumulated 4-hydroxy-3-methoxyphenyl-β-ketopropionate in the culture supernatant when incubated with ferulate, supporting β-oxidative deacetylation. This phenotype was not complemented with a D257N variant of CouO, consistent with the predicted role of Asp257 as a metal ligand in this amidohydrolase superfamily member. These data suggest that CouO functionally replaces the β-ketothiolase and acyl-CoA thioesterase that occur in canonical β-oxidative pathways. Finally, the transcriptomics data suggest the involvement of two distinct formaldehyde detoxification pathways in vanillate catabolism and identify a eugenol catabolic pathway. The results of this study augment our understanding of the bacterial catabolism of aromatics from renewable feedstocks.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures



Similar articles
-
Functional Redundancy in the Hydroxycinnamate Catabolism Pathways of the Salt Marsh Bacterium Sagittula stellata E-37.Appl Environ Microbiol. 2018 Nov 15;84(23):e02027-18. doi: 10.1128/AEM.02027-18. Print 2018 Dec 1. Appl Environ Microbiol. 2018. PMID: 30242006 Free PMC article.
-
The catabolism of ethylene glycol by Rhodococcus jostii RHA1 and its dependence on mycofactocin.Appl Environ Microbiol. 2024 Jul 24;90(7):e0041624. doi: 10.1128/aem.00416-24. Epub 2024 Jun 5. Appl Environ Microbiol. 2024. PMID: 38837369 Free PMC article.
-
Vanillin catabolism in Rhodococcus jostii RHA1.Appl Environ Microbiol. 2012 Jan;78(2):586-8. doi: 10.1128/AEM.06876-11. Epub 2011 Nov 4. Appl Environ Microbiol. 2012. PMID: 22057861 Free PMC article.
-
Biotechnological Potential of Rhodococcus Biodegradative Pathways.J Microbiol Biotechnol. 2018 Jul 28;28(7):1037-1051. doi: 10.4014/jmb.1712.12017. J Microbiol Biotechnol. 2018. PMID: 29913546 Review.
-
Modification of plant cell walls with hydroxycinnamic acids by BAHD acyltransferases.Front Plant Sci. 2023 Jan 17;13:1088879. doi: 10.3389/fpls.2022.1088879. eCollection 2022. Front Plant Sci. 2023. PMID: 36733587 Free PMC article. Review.
Cited by
-
Applying biochemical and structural characterization of hydroxycinnamate catabolic enzymes from soil metagenome for lignin valorization strategies.Appl Microbiol Biotechnol. 2022 Apr;106(7):2503-2516. doi: 10.1007/s00253-022-11885-3. Epub 2022 Mar 30. Appl Microbiol Biotechnol. 2022. PMID: 35352150
-
IpdE1-IpdE2 Is a Heterotetrameric Acyl Coenzyme A Dehydrogenase That Is Widely Distributed in Steroid-Degrading Bacteria.Biochemistry. 2020 Mar 17;59(10):1113-1123. doi: 10.1021/acs.biochem.0c00005. Epub 2020 Mar 4. Biochemistry. 2020. PMID: 32101684 Free PMC article.
-
The Operon Encoding Hydrolytic Dehalogenation of 4-Chlorobenzoate Is Transcriptionally Regulated by the TetR-Type Repressor FcbR and Its Ligand 4-Chlorobenzoyl Coenzyme A.Appl Environ Microbiol. 2021 Feb 26;87(6):e02652-20. doi: 10.1128/AEM.02652-20. Print 2021 Feb 26. Appl Environ Microbiol. 2021. PMID: 33397703 Free PMC article.
-
The Catabolic System of Acetovanillone and Acetosyringone in Sphingobium sp. Strain SYK-6 Useful for Upgrading Aromatic Compounds Obtained through Chemical Lignin Depolymerization.Appl Environ Microbiol. 2022 Aug 23;88(16):e0072422. doi: 10.1128/aem.00724-22. Epub 2022 Aug 8. Appl Environ Microbiol. 2022. PMID: 35938864 Free PMC article.
-
Mapping the diversity of microbial lignin catabolism: experiences from the eLignin database.Appl Microbiol Biotechnol. 2019 May;103(10):3979-4002. doi: 10.1007/s00253-019-09692-4. Epub 2019 Apr 8. Appl Microbiol Biotechnol. 2019. PMID: 30963208 Free PMC article. Review.
References
-
- Mialon L, Pemba GP, Miller SH. 2010. Biorenewable polyethylene terephthalate mimics derived from lignin and acetic acid. Green Chem. 12:1704–1706. 10.1039/c0gc00150c. - DOI
-
- Yam KC, van der Geize R, Eltis LD. 2010. Catabolism of aromatic compounds and steroids by Rhodococcus, p 133–169 In Alvarez HM. (ed), Biology of Rhodococcus. Springer-Verlag, Berlin, Germany.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

