Coregulated expression of the Na+/phosphate Pho89 transporter and Ena1 Na+-ATPase allows their functional coupling under high-pH stress
- PMID: 25266663
- PMCID: PMC4248728
- DOI: 10.1128/MCB.01089-14
Coregulated expression of the Na+/phosphate Pho89 transporter and Ena1 Na+-ATPase allows their functional coupling under high-pH stress
Abstract
The yeast Saccharomyces cerevisiae has two main high-affinity inorganic phosphate (Pi) transporters, Pho84 and Pho89, that are functionally relevant at acidic/neutral pH and alkaline pH, respectively. Upon Pi starvation, PHO84 and PHO89 are induced by the activation of the PHO regulon by the binding of the Pho4 transcription factor to specific promoter sequences. We show that PHO89 and PHO84 are induced by alkalinization of the medium with different kinetics and that the network controlling Pho89 expression in response to alkaline pH differs from that of other members of the PHO regulon. In addition to Pho4, the PHO89 promoter is regulated by the transcriptional activator Crz1 through the calcium-activated phosphatase calcineurin, and it is under the control of several repressors (Mig2, Nrg1, and Nrg2) coordinately regulated by the Snf1 protein kinase and the Rim101 transcription factor. This network mimics the one regulating expression of the Na(+)-ATPase gene ENA1, encoding a major determinant for Na(+) detoxification. Our data highlight a scenario in which the activities of Pho89 and Ena1 are functionally coordinated to sustain growth in an alkaline environment.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures

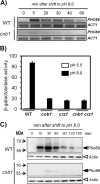



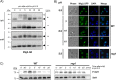
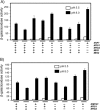

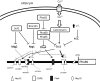
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
