Hyaluronan synthase 2 protects skin fibroblasts against apoptosis induced by environmental stress
- PMID: 25266724
- PMCID: PMC4231699
- DOI: 10.1074/jbc.M114.578377
Hyaluronan synthase 2 protects skin fibroblasts against apoptosis induced by environmental stress
Abstract
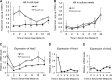
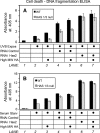
A balanced turnover of dermal fibroblasts is crucial for structural integrity and normal function of the skin. During recovery from environmental injury (such as UV exposure and physical wounding), apoptosis is an important mechanism regulating fibroblast turnover. We are interested in the role that hyaluronan (HA), an extracellular matrix molecule synthesized by HA synthase enzymes (Has), plays in regulating apoptosis in fibroblasts. We previously reported that Has1 and Has3 double knock-out (Has1/3 null) mice show accelerated wound closure and increased numbers of fibroblasts in the dermis. In the present study, we report that HA levels and Has2 mRNA expression are higher in cultured Has1/3 null primary skin fibroblasts than in wild type (WT) cells. Apoptosis induced by two different environmental stressors, UV exposure and serum starvation (SS), was reduced in the Has1/3 null cells. Hyaluronidase, added to cultures to remove extracellular HA, surprisingly had no effect upon apoptotic susceptibility to UVB or SS. However, cells treated with 4-methylumbelliferone to inhibit HA synthesis were sensitized to apoptosis induced by SS or UVB. When fibroblasts were transfected with Has2-specific siRNA that lowered Has2 mRNA and HA levels by 90%, both Has1/3 null and WT cells became significantly more sensitive to apoptosis. The exogenous addition of high molecular weight HA failed to reverse this effect. We conclude that Has1/3 null skin fibroblasts (which have higher levels of Has2 gene expression) are resistant to stress-induced apoptosis.
Keywords: Apoptosis; Caspase; Extracellular Matrix; Fibroblast; Hyaluronan; Skin; Stress.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures







References
-
- Kubo K., Kuroyanagi Y. (2005) A study of cytokines released from fibroblasts in cultured dermal substitute. Artif. Organs 29, 845–849 - PubMed
-
- Wong T., McGrath J. A., Navsaria H. (2007) The role of fibroblasts in tissue engineering and regeneration. Br. J. Dermatol. 156, 1149–1155 - PubMed
-
- Werner S., Krieg T., Smola H. (2007) Keratinocyte-fibroblast interactions in wound healing. J. Invest. Dermatol. 127, 998–1008 - PubMed
-
- Jelaska A., Korn J. H. (2000) Role of apoptosis and transforming growth factor β1 in fibroblast selection and activation in systemic sclerosis. Arthritis Rheum. 43, 2230–2239 - PubMed
-
- Jun J. B., Kuechle M., Min J., Shim S. C., Kim G., Montenegro V., Korn J. H., Elkon K. B. (2005) Scleroderma fibroblasts demonstrate enhanced activation of Akt (protein kinase B) in situ. J. Invest. Dermatol. 124, 298–303 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

