Marine algae and land plants share conserved phytochrome signaling systems
- PMID: 25267653
- PMCID: PMC4226090
- DOI: 10.1073/pnas.1416751111
Marine algae and land plants share conserved phytochrome signaling systems
Abstract
Phytochrome photosensors control a vast gene network in streptophyte plants, acting as master regulators of diverse growth and developmental processes throughout the life cycle. In contrast with their absence in known chlorophyte algal genomes and most sequenced prasinophyte algal genomes, a phytochrome is found in Micromonas pusilla, a widely distributed marine picoprasinophyte (<2 µm cell diameter). Together with phytochromes identified from other prasinophyte lineages, we establish that prasinophyte and streptophyte phytochromes share core light-input and signaling-output domain architectures except for the loss of C-terminal response regulator receiver domains in the streptophyte phytochrome lineage. Phylogenetic reconstructions robustly support the presence of phytochrome in the common progenitor of green algae and land plants. These analyses reveal a monophyletic clade containing streptophyte, prasinophyte, cryptophyte, and glaucophyte phytochromes implying an origin in the eukaryotic ancestor of the Archaeplastida. Transcriptomic measurements reveal diurnal regulation of phytochrome and bilin chromophore biosynthetic genes in Micromonas. Expression of these genes precedes both light-mediated phytochrome redistribution from the cytoplasm to the nucleus and increased expression of photosynthesis-associated genes. Prasinophyte phytochromes perceive wavelengths of light transmitted farther through seawater than the red/far-red light sensed by land plant phytochromes. Prasinophyte phytochromes also retain light-regulated histidine kinase activity lost in the streptophyte phytochrome lineage. Our studies demonstrate that light-mediated nuclear translocation of phytochrome predates the emergence of land plants and likely represents a widespread signaling mechanism in unicellular algae.
Keywords: light harvesting; light signaling evolution; marine ecology; phytoplankton; transcriptomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

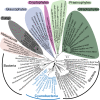


Comment in
-
Algae hold clues to eukaryotic origins of plant phytochromes.Proc Natl Acad Sci U S A. 2014 Nov 4;111(44):15608-9. doi: 10.1073/pnas.1417990111. Epub 2014 Oct 27. Proc Natl Acad Sci U S A. 2014. PMID: 25349430 Free PMC article. No abstract available.
References
-
- Chen M, Chory J, Fankhauser C. Light signal transduction in higher plants. Annu Rev Genet. 2004;38:87–117. - PubMed
-
- Giraud E, Verméglio A. Bacteriophytochromes in anoxygenic photosynthetic bacteria. Photosynth Res. 2008;97(2):141–153. - PubMed
-
- Rodriguez-Romero J, Hedtke M, Kastner C, Müller S, Fischer R. Fungi, hidden in soil or up in the air: Light makes a difference. Annu Rev Microbiol. 2010;64:585–610. - PubMed
-
- Auldridge ME, Forest KT. Bacterial phytochromes: More than meets the light. Crit Rev Biochem Mol Biol. 2011;46(1):67–88. - PubMed
-
- Mathews S. Phytochrome-mediated development in land plants: Red light sensing evolves to meet the challenges of changing light environments. Mol Ecol. 2006;15(12):3483–3503. - PubMed
Publication types
MeSH terms
Substances
Associated data
- BioProject/PRJNA231566
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

