Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning
- PMID: 25267759
- PMCID: PMC4209101
- DOI: 10.1200/JCO.2013.54.0625
Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning
Abstract
Purpose: The clinical safety and efficacy of intravenous busulfan and fludarabine (IV Bu/Flu) myeloablative conditioning as well as graft-versus-host disease (GVHD) prophylaxis with high-dose, post-transplantation cyclophosphamide (PTCy) have been demonstrated independently in several single-institutional studies. We hypothesized that combining these two promising approaches in a multi-institutional study of human leukocyte antigen (HLA) -matched bone marrow transplantation would provide low rates of severe acute and chronic GVHD, low toxicity, and effective disease control.
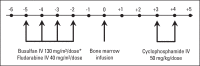
Patients and methods: Ninety-two adult patients (median age, 49 years; range, 21 to 65 years) with high-risk hematologic malignancies were enrolled at three centers (clinical trial No. NCT00809276). Forty-five patients received related allografts, and 47 received unrelated allografts. GVHD prophylaxis was solely with PTCy at 50 mg/kg/day on post-transplantation days +3 and +4.
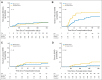
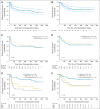
Results: The cumulative incidences of grades 2 to 4 acute, grades 3 to 4 acute, and chronic GVHD were 51%, 15%, and 14%, respectively. Nonrelapse mortality (NRM) at 100 days and 1 year were 9% and 16%, respectively. With a median follow-up period of 2.2 years, the 2-year disease-free survival (DFS) and overall survival (OS) rates were 62% and 67%, respectively. Donor relatedness did not affect NRM, DFS, or OS. Patients in complete remission (CR) without evidence of minimal residual disease (MRD) had markedly better DFS (80%) and OS (80%) than patients in CR with MRD or with active disease at the time of transplantation (DFS, P = .0005; OS, P = .019).
Conclusion: This multi-institutional study demonstrates that PTCy can be safely and effectively combined with IV Bu/Flu myeloablative conditioning and confirms PTCy's efficacy as single-agent, short-course GVHD prophylaxis for both acute and chronic GVHD after bone marrow transplantation from HLA-matched donors.
© 2014 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Horwitz ME. Reduced intensity versus myeloablative allogeneic stem cell transplantation for the treatment of acute myeloid leukemia, myelodysplastic syndrome and acute lymphoid leukemia. Curr Opin Oncol. 2011;23:197–202. - PubMed
-
- Couban S, Simpson DR, Barnett MJ, et al. A randomized multicenter comparison of bone marrow and peripheral blood in recipients of matched sibling allogeneic transplants for myeloid malignancies. Blood. 2002;100:1525–1531. - PubMed
-
- Bacigalupo A, Van Lint MT, Occhini D, et al. Increased risk of leukemia relapse with high-dose cyclosporine A after allogeneic marrow transplantation for acute leukemia. Blood. 1991;77:1423–1428. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

