CARD9 mediates Dectin-1-induced ERK activation by linking Ras-GRF1 to H-Ras for antifungal immunity
- PMID: 25267792
- PMCID: PMC4203953
- DOI: 10.1084/jem.20132349
CARD9 mediates Dectin-1-induced ERK activation by linking Ras-GRF1 to H-Ras for antifungal immunity
Abstract
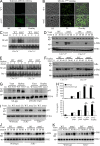
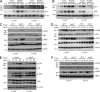
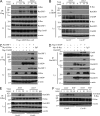
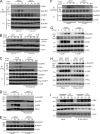
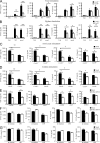
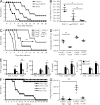
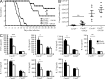
Dectin-1 functions as a pattern recognition receptor for sensing fungal infection. It has been well-established that Dectin-1 induces innate immune responses through caspase recruitment domain-containing protein 9 (CARD9)-mediated NF-κB activation. In this study, we find that CARD9 is dispensable for NF-κB activation induced by Dectin-1 ligands, such as curdlan or Candida albicans yeast. In contrast, we find that CARD9 regulates H-Ras activation by linking Ras-GRF1 to H-Ras, which mediates Dectin-1-induced extracellular signal-regulated protein kinase (ERK) activation and proinflammatory responses when stimulated by their ligands. Mechanistically, Dectin-1 engagement initiates spleen tyrosine kinase (Syk)-dependent Ras-GRF1 phosphorylation, and the phosphorylated Ras-GRF1 recruits and activates H-Ras through forming a complex with CARD9, which leads to activation of ERK downstream. Finally, we show that inhibiting ERK activation significantly accelerates the death of C. albicans-infected mice, and this inhibitory effect is dependent on CARD9. Together, our studies reveal a molecular mechanism by which Dectin-1 induces H-Ras activation that leads to ERK activation for host innate immune responses against fungal infection.
© 2014 Jia et al.
Figures

References
-
- Bai, C., Xu X.L., Chan F.Y., Lee R.T.H., and Wang Y.. 2006. MNN5 encodes an iron-regulated α-1,2-mannosyltransferase important for protein glycosylation, cell wall integrity, morphogenesis, and virulence in Candida albicans. Eukaryot. Cell. 5:238–247. 10.1128/EC.5.2.238-247.2006 - DOI - PMC - PubMed
-
- Bertin, J., Guo Y., Wang L., Srinivasula S.M., Jacobson M.D., Poyet J.-L., Merriam S., Du M.-Q., Dyer M.J., Robison K.E., et al. . 2000. CARD9 is a novel caspase recruitment domain-containing protein that interacts with BCL10/CLAP and activates NF-κB. J. Biol. Chem. 275:41082–41086. 10.1074/jbc.C000726200 - DOI - PubMed
-
- Bi, L., Gojestani S., Wu W., Hsu Y.M.S., Zhu J., Ariizumi K., and Lin X.. 2010. CARD9 mediates dectin-2-induced IκBα kinase ubiquitination leading to activation of NF-κB in response to stimulation by the hyphal form of Candida albicans. J. Biol. Chem. 285:25969–25977. 10.1074/jbc.M110.131300 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

