Inflammasome activation by Campylobacter jejuni
- PMID: 25267974
- PMCID: PMC4201959
- DOI: 10.4049/jimmunol.1400648
Inflammasome activation by Campylobacter jejuni
Abstract
The Gram-negative pathogen Campylobacter jejuni is the most common cause of bacterial foodborne disease worldwide. The mechanisms that lead to bacterial invasion of eukaryotic cells and massive intestinal inflammation are still unknown. In this study, we report that C. jejuni infection of mouse macrophages induces upregulation of pro-IL-1β transcript and secretion of IL-1β without eliciting cell death. Immunoblotting indicated cleavage of caspase-1 and IL-1β in infected cells. In bone marrow-derived macrophages from different knockout mice, IL-1β secretion was found to require NLRP3, ASC, and caspase-1/11 but not NLRC4. In contrast to NLRP3 activation by ATP, C. jejuni activation did not require priming of these macrophages. C. jejuni also activated the NLRP3 inflammasome in human macrophages as indicated by the presence of ASC foci and caspase-1-positive cells. Analysis of a vast array of C. jejuni mutants with defects in capsule formation, LPS biosynthesis, chemotaxis, flagella synthesis and flagellin (-like) secretion, type 6 secretion system needle protein, or cytolethal distending toxin revealed a direct correlation between the number of intracellular bacteria and NLRP3 inflammasome activation. The C. jejuni invasion-related activation of the NLRP3 inflammasome without cytotoxicity and even in nonprimed cells extends the known repertoire of bacterial inflammasome activation and likely contributes to C. jejuni-induced intestinal inflammation.
Copyright © 2014 by The American Association of Immunologists, Inc.
Figures

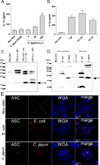
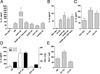



References
-
- Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356:768–774. - PubMed
-
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-1beta. Mol. Cell. 2002;10:417–426. - PubMed
-
- Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

