The architecture of CCAN proteins creates a structural integrity to resist spindle forces and achieve proper Intrakinetochore stretch
- PMID: 25268173
- PMCID: PMC4237209
- DOI: 10.1016/j.devcel.2014.08.003
The architecture of CCAN proteins creates a structural integrity to resist spindle forces and achieve proper Intrakinetochore stretch
Abstract
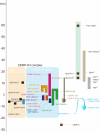
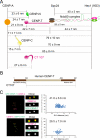
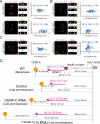
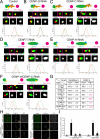
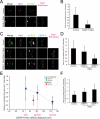
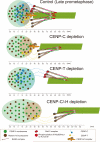
Constitutive centromere-associated network (CCAN) proteins, particularly CENP-C, CENP-T, and the CENP-H/-I complex, mechanically link CENP-A-containing centromeric chromatin within the inner kinetochore to outer kinetochore proteins, such as the Ndc80 complex, that bind kinetochore microtubules. Accuracy of chromosome segregation depends critically upon Aurora B phosphorylation of Ndc80/Hec1. To determine how CCAN protein architecture mechanically constrains intrakinetochore stretch between CENP-A and Ndc80/Hec1 for proper Ndc80/Hec1 phosphorylation, we used super-resolution fluorescence microscopy and selective protein depletion. We found that at bi-oriented chromosomes in late prometaphase cells, CENP-T is stretched ∼16 nm to the inner end of Ndc80/Hec1, much less than expected for full-length CENP-T. Depletion of various CCAN linker proteins induced hyper-intrakinetochore stretch (an additional 20-60 nm) with corresponding significant decreases in Aurora B phosphorylation of Ndc80/Hec1. Thus, proper intrakinetochore stretch is required for normal kinetochore function and depends critically on all the CCAN mechanical linkers to the Ndc80 complex.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

