Selecting first-line bevacizumab-containing therapy for advanced breast cancer: TURANDOT risk factor analyses
- PMID: 25268370
- PMCID: PMC4260030
- DOI: 10.1038/bjc.2014.504
Selecting first-line bevacizumab-containing therapy for advanced breast cancer: TURANDOT risk factor analyses
Abstract
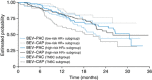
Background: The randomised phase III TURANDOT trial compared first-line bevacizumab-paclitaxel (BEV-PAC) vs bevacizumab-capecitabine (BEV-CAP) in HER2-negative locally recurrent/metastatic breast cancer (LR/mBC). The interim analysis revealed no difference in overall survival (OS; primary end point) between treatment arms; however, progression-free survival (PFS) and objective response rate were significantly superior with BEV-PAC. We sought to identify patient populations that may be most appropriately treated with one or other regimen.
Methods: Patients with HER2-negative LR/mBC who had received no prior chemotherapy for advanced disease were randomised to either BEV-PAC (bevacizumab 10 mg kg(-1) days 1 and 15 plus paclitaxel 90 mg m(-2) days 1, 8 and 15 q4w) or BEV-CAP (bevacizumab 15 mg kg(-1) day 1 plus capecitabine 1000 mg m(-2) bid days 1-14 q3w). The study population was categorised into three cohorts: triple-negative breast cancer (TNBC), high-risk hormone receptor-positive (HR+) and low-risk HR+. High- and low-risk HR+ were defined, respectively, as having ⩾2 vs ⩽1 of the following four risk factors: disease-free interval ⩽24 months; visceral metastases; prior (neo)adjuvant anthracycline and/or taxane; and metastases in ⩾3 organs.
Results: The treatment effect on OS differed between cohorts. Non-significant OS trends favoured BEV-PAC in the TNBC cohort and BEV-CAP in the low-risk HR+ cohort. In all three cohorts, there was a non-significant PFS trend favouring BEV-PAC. Grade ⩾3 adverse events were consistently less common with BEV-CAP.
Conclusions: A simple risk factor index may help in selecting bevacizumab-containing regimens, balancing outcome, safety profile and patient preference. Final OS results are expected in 2015 (ClinicalTrials.gov NCT00600340).
Figures

References
-
- Baselga J, Gómez P, Greil R, Braga S, Climent MA, Wardley AM, Kaufman B, Stemmer SM, Pêgo A, Chan A, Goeminne JC, Graas MP, Kennedy MJ, Ciruelos Gil EM, Schneeweiss A, Zubel A, Groos J, Melezínková H, Awada A. Randomized phase II study of the anti-epidermal growth factor receptor monoclonal antibody cetuximab with cisplatin versus cisplatin alone in patients with metastatic triple-negative breast cancer. J Clin Oncol. 2013;31:2586–2592. - PMC - PubMed
-
- Brufsky A, Valero V, Tiangco B, Dakhil S, Brize A, Rugo HS, Rivera R, Duenne A, Bousfoul N, Yardley DA. Second-line bevacizumab-containing therapy in patients with triple-negative breast cancer: subgroup analysis of the RIBBON-2 trial. Breast Cancer Res Treat. 2012;133:1067–1075. - PubMed
-
- Dawood S, Shaikh AJ, Buchholz TA, Cortes J, Cristofanilli M, Gupta S, Gonzalez-Angulo AM. The use of bevacizumab among women with metastatic breast cancer: a survey on clinical practice and the ongoing controversy. Cancer. 2012;118:2780–2786. - PubMed
-
- Inbar MJ, Lang I, Kahan Z, Greil R, Beslija S, Stemmer SM, Kaufman B, Ahlers S, Brodowicz T, Zielinski C, Central European Cooperative Oncology Group Efficacy of first-line bevacizumab (BEV)-based therapy for metastatic triple-negative breast cancer (TNBC): subgroup analysis of TURANDOT. J Clin Oncol. 2013;31 (suppl:abstract 1040.
-
- Lang I, Brodowicz T, Ryvo L, Kahan Z, Greil R, Beslija S, Stemmer SM, Kaufman B, Zvirbule Z, Steger GG, Melichar B, Pienkowski T, Sirbu D, Messinger D, Zielinski C, Central European Cooperative Oncology Group Bevacizumab plus paclitaxel versus bevacizumab plus capecitabine as first-line treatment for HER2-negative metastatic breast cancer: interim efficacy results of the randomised, open-label, non-inferiority, phase 3 TURANDOT trial. Lancet Oncol. 2013;14:125–133. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

