New developmental evidence clarifies the evolution of wrist bones in the dinosaur-bird transition
- PMID: 25268520
- PMCID: PMC4181957
- DOI: 10.1371/journal.pbio.1001957
New developmental evidence clarifies the evolution of wrist bones in the dinosaur-bird transition
Abstract
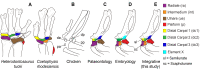
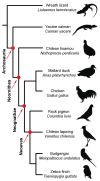
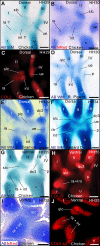
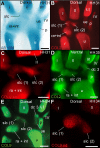
From early dinosaurs with as many as nine wrist bones, modern birds evolved to develop only four ossifications. Their identity is uncertain, with different labels used in palaeontology and developmental biology. We examined embryos of several species and studied chicken embryos in detail through a new technique allowing whole-mount immunofluorescence of the embryonic cartilaginous skeleton. Beyond previous controversy, we establish that the proximal-anterior ossification develops from a composite radiale+intermedium cartilage, consistent with fusion of radiale and intermedium observed in some theropod dinosaurs. Despite previous claims that the development of the distal-anterior ossification does not support the dinosaur-bird link, we found its embryonic precursor shows two distinct regions of both collagen type II and collagen type IX expression, resembling the composite semilunate bone of bird-like dinosaurs (distal carpal 1+distal carpal 2). The distal-posterior ossification develops from a cartilage referred to as "element x," but its position corresponds to distal carpal 3. The proximal-posterior ossification is perhaps most controversial: It is labelled as the ulnare in palaeontology, but we confirm the embryonic ulnare is lost during development. Re-examination of the fossil evidence reveals the ulnare was actually absent in bird-like dinosaurs. We confirm the proximal-posterior bone is a pisiform in terms of embryonic position and its development as a sesamoid associated to a tendon. However, the pisiform is absent in bird-like dinosaurs, which are known from several articulated specimens. The combined data provide compelling evidence of a remarkable evolutionary reversal: A large, ossified pisiform re-evolved in the lineage leading to birds, after a period in which it was either absent, nonossified, or very small, consistently escaping fossil preservation. The bird wrist provides a modern example of how developmental and paleontological data illuminate each other. Based on all available data, we introduce a new nomenclature for bird wrist ossifications.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

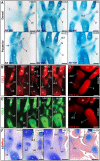

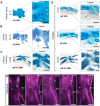
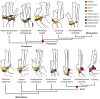
Comment in
-
Resolving the flap over bird wrists.PLoS Biol. 2014 Sep 30;12(9):e1001958. doi: 10.1371/journal.pbio.1001958. eCollection 2014 Sep. PLoS Biol. 2014. PMID: 25268645 Free PMC article.
Similar articles
-
Reorganization of the theropod wrist preceded the origin of avian flight.Nature. 2025 Aug;644(8077):699-705. doi: 10.1038/s41586-025-09232-3. Epub 2025 Jul 9. Nature. 2025. PMID: 40634603
-
Do feathered dinosaurs exist? Testing the hypothesis on neontological and paleontological evidence.J Morphol. 2005 Nov;266(2):125-66. doi: 10.1002/jmor.10382. J Morphol. 2005. PMID: 16217748
-
The asymmetry of the carpal joint and the evolution of wing folding in maniraptoran theropod dinosaurs.Proc Biol Sci. 2010 Jul 7;277(1690):2027-33. doi: 10.1098/rspb.2009.2281. Epub 2010 Mar 3. Proc Biol Sci. 2010. PMID: 20200032 Free PMC article.
-
Tracing the evolution of avian wing digits.Curr Biol. 2013 Jun 17;23(12):R538-44. doi: 10.1016/j.cub.2013.04.071. Curr Biol. 2013. PMID: 23787052 Free PMC article. Review.
-
The evolutionary continuum of limb function from early theropods to birds.Naturwissenschaften. 2009 Apr;96(4):423-48. doi: 10.1007/s00114-008-0488-3. Epub 2008 Dec 24. Naturwissenschaften. 2009. PMID: 19107456 Review.
Cited by
-
A Problematic Tyrannosaurid (Dinosauria: Theropoda) Skeleton and Its Implications for Tyrannosaurid Diversity in the Horseshoe Canyon Formation (Upper Cretaceous) of Alberta.Anat Rec (Hoboken). 2020 Apr;303(4):673-690. doi: 10.1002/ar.24199. Epub 2019 Jun 29. Anat Rec (Hoboken). 2020. PMID: 31254458 Free PMC article.
-
Hand/foot splitting and the 're-evolution' of mesopodial skeletal elements during the evolution and radiation of chameleons.BMC Evol Biol. 2015 Sep 18;15:184. doi: 10.1186/s12862-015-0464-4. BMC Evol Biol. 2015. PMID: 26382964 Free PMC article.
-
Phylogeny of Paleozoic limbed vertebrates reassessed through revision and expansion of the largest published relevant data matrix.PeerJ. 2019 Jan 4;6:e5565. doi: 10.7717/peerj.5565. eCollection 2019. PeerJ. 2019. PMID: 30631641 Free PMC article.
-
A non-archaeopterygid avialan theropod from the Late Jurassic of southern Germany.Elife. 2019 May 14;8:e43789. doi: 10.7554/eLife.43789. Elife. 2019. PMID: 31084702 Free PMC article.
-
Forty new specimens of Ichthyornis provide unprecedented insight into the postcranial morphology of crownward stem group birds.PeerJ. 2022 Dec 16;10:e13919. doi: 10.7717/peerj.13919. eCollection 2022. PeerJ. 2022. PMID: 36545383 Free PMC article.
References
-
- Santa Luca A (1980) The postcranial skeleton of Heterodontosaurus tucki (Reptilia, Ornithischia) from the Stromberg of South Africa. Ann S Afr Mus 79: 159–211.
-
- Richardson M (2012) Manus horribilis: the chicken wing skeleton. In: RJ A, J M, editors. From clone to bone: the synergy of morphological and molecular tools in palaeobiology. Cambridge, UK: Cambridge University Press. pp. 328–362.
-
- Carrano MT, Benson RBJ, Sampson SD (2012) The phylogeny of Tetanurae (Dinosauria: Theropoda). J Syst Palaeontol 10: 211–300.
-
- Gauthier J (1986) Saurischian monophyly and the origin of birds. Mem Calif Acad Sci 8: 1–55.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

