Transcript profiling of structural genes involved in cyanidin-based anthocyanin biosynthesis between purple and non-purple carrot (Daucus carota L.) cultivars reveals distinct patterns
- PMID: 25269413
- PMCID: PMC4190390
- DOI: 10.1186/s12870-014-0262-y
Transcript profiling of structural genes involved in cyanidin-based anthocyanin biosynthesis between purple and non-purple carrot (Daucus carota L.) cultivars reveals distinct patterns
Abstract
Background: Carrots (Daucus carota L.) are among the 10 most economically important vegetable crops grown worldwide. Purple carrot cultivars accumulate rich cyanidin-based anthocyanins in a light-independent manner in their taproots whereas other carrot color types do not. Anthocyanins are important secondary metabolites in plants, protecting them from damage caused by strong light, heavy metals, and pathogens. Furthermore, they are important nutrients for human health. Molecular mechanisms underlying anthocyanin accumulation in purple carrot cultivars and loss of anthocyanin production in non-purple carrot cultivars remain unknown.
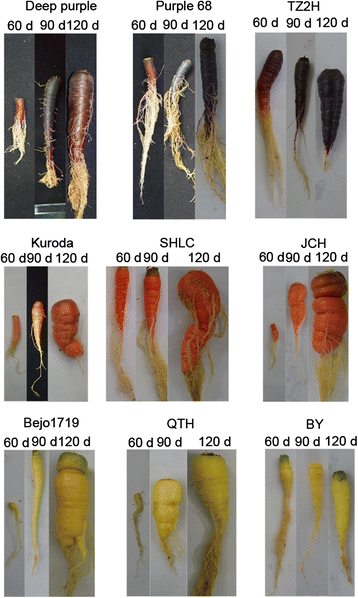
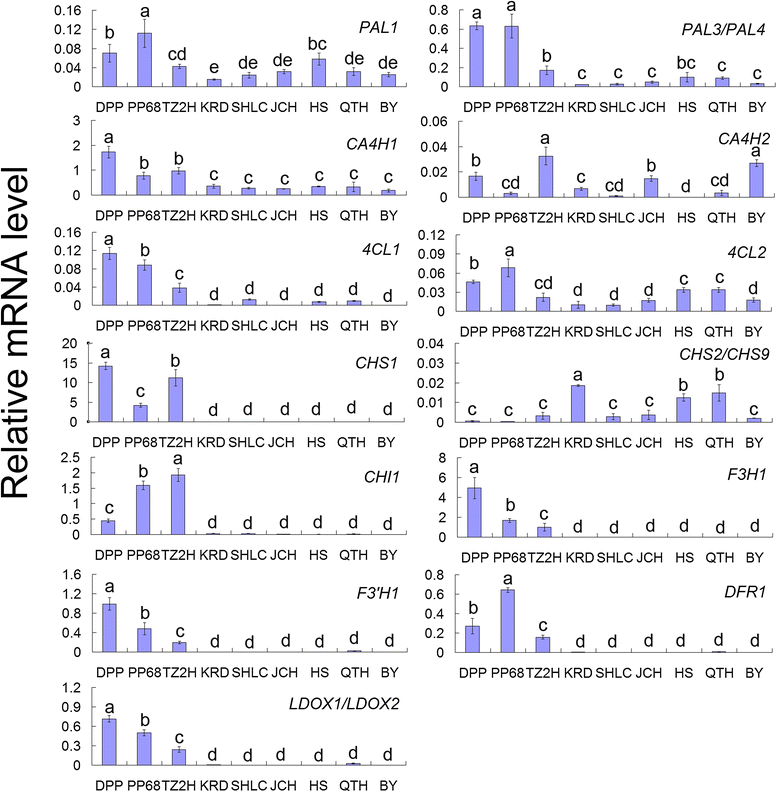
Results: The taproots of the three purple carrot cultivars were rich in anthocyanin, and levels increased during development. Conversely, the six non-purple carrot cultivars failed to accumulate anthocyanins in the underground part of taproots. Six novel structural genes, CA4H1, CA4H2, 4CL1, 4CL2, CHI1, and F3'H1, were isolated from purple carrots. The expression profiles of these genes, together with other structural genes known to be involved in anthocyanin biosynthesis, were analyzed in three purple and six non-purple carrot cultivars at the 60-day-old stage. PAL3/PAL4, CA4H1, and 4CL1 expression levels were higher in purple than in non-purple carrot cultivars. Expression of CHS1, CHI1, F3H1, F3'H1, DFR1, and LDOX1/LDOX2 was highly correlated with the presence of anthocyanin as these genes were highly expressed in purple carrot taproots but not or scarcely expressed in non-purple carrot taproots.
Conclusions: This study isolated six novel structural genes involved in anthocyanin biosynthesis in carrots. Among the 13 analyzed structural genes, PAL3/PAL4, CA4H1, 4CL1, CHS1, CHI1, F3H1, F3'H1, DFR1, and LDOX1/LDOX2 may participate in anthocyanin biosynthesis in the taproots of purple carrot cultivars. CHS1, CHI1, F3H1, F3'H1, DFR1, and LDOX1/LDOX2 may lead to loss of light-independent anthocyanin production in orange and yellow carrots. These results suggest that numerous structural genes are involved in anthocyanin production in the taproots of purple carrot cultivars and in the loss of anthocyanin production in non-purple carrots. Unexpressed or scarcely expressed genes in the taproots of non-purple carrot cultivars may be caused by the inactivation of regulator genes. Our results provide new insights into anthocyanin biosynthesis at the molecular level in carrots and for other root vegetables.
Figures



References
-
- Shirley BW. Flavonoids in seeds and grains: physiological function, agronomic importance and the genetics of biosynthesis. Seed Sci Res. 1998;8(04):415–422. doi: 10.1017/S0960258500004372. - DOI
-
- Bąkowska-Barczak A. Acylated anthocyanins as stable, natural food colorants–a review. Int J Food Sci Nutr. 2005;14(2):107–116.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

