MicroRNA-193a Regulates the Transdifferentiation of Human Parietal Epithelial Cells toward a Podocyte Phenotype
- PMID: 25270065
- PMCID: PMC4446868
- DOI: 10.1681/ASN.2014020190
MicroRNA-193a Regulates the Transdifferentiation of Human Parietal Epithelial Cells toward a Podocyte Phenotype
Abstract
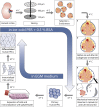
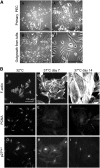
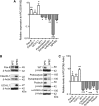
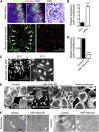
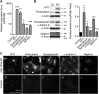
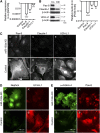
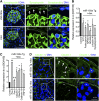
Parietal epithelial cells have been identified as potential progenitor cells in glomerular regeneration, but the molecular mechanisms underlying this process are not fully defined. Here, we established an immortalized polyclonal human parietal epithelial cell (hPEC) line from naive human Bowman's capsule cells isolated by mechanical microdissection. These hPECs expressed high levels of PEC-specific proteins and microRNA-193a (miR-193a), a suppressor of podocyte differentiation through downregulation of Wilms' tumor 1 in mice. We then investigated the function of miR-193a in the establishment of podocyte and PEC identity and determined whether inhibition of miR-193a influences the behavior of PECs in glomerular disease. After stable knockdown of miR-193a, hPECs adopted a podocyte-like morphology and marker expression, with decreased expression levels of PEC markers. In mice, inhibition of miR-193a by complementary locked nucleic acids resulted in an upregulation of the podocyte proteins synaptopodin and Wilms' tumor 1. Conversely, overexpression of miR-193a in vivo resulted in the upregulation of PEC markers and the loss of podocyte markers in isolated glomeruli. Inhibition of miR-193a in a mouse model of nephrotoxic nephritis resulted in reduced crescent formation and decreased proteinuria. Together, these results show the establishment of a human PEC line and suggest that miR-193a functions as a master switch, such that glomerular epithelial cells with high levels of miR-193a adopt a PEC phenotype and cells with low levels of miR-193a adopt a podocyte phenotype. miR-193a-mediated maintenance of PECs in an undifferentiated reactive state might be a prerequisite for PEC proliferation and migration in crescent formation.
Keywords: expression; podocyte; renal epithelial cell.
Copyright © 2015 by the American Society of Nephrology.
Figures


Comment in
-
Podocyte biology: Differentiation of parietal epithelial cells into podocytes.Nat Rev Nephrol. 2015 Jan;11(1):7-8. doi: 10.1038/nrneph.2014.218. Epub 2014 Nov 25. Nat Rev Nephrol. 2015. PMID: 25421831 No abstract available.
References
-
- Shankland SJ, Smeets B, Pippin JW, Moeller MJ: The emergence of the glomerular parietal epithelial cell. Nat Rev Nephrol 10: 158–173, 2014 - PubMed
-
- Discenza MT, He S, Lee TH, Chu LL, Bolon B, Goodyer P, Eccles M, Pelletier J: WT1 is a modifier of the Pax2 mutant phenotype: Cooperation and interaction between WT1 and Pax2. Oncogene 22: 8145–8155, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

