Specific macrophage subtypes influence the progression of rhabdomyolysis-induced kidney injury
- PMID: 25270069
- PMCID: PMC4446873
- DOI: 10.1681/ASN.2014040320
Specific macrophage subtypes influence the progression of rhabdomyolysis-induced kidney injury
Abstract
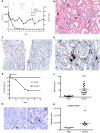
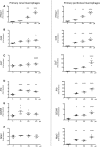
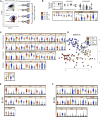
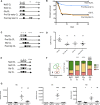
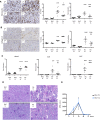
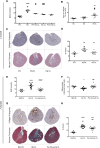
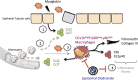
Rhabdomyolysis can be life threatening if complicated by AKI. Macrophage infiltration has been observed in rat kidneys after glycerol-induced rhabdomyolysis, but the role of macrophages in rhabdomyolysis-induced AKI remains unknown. Here, in a patient diagnosed with rhabdomyolysis, we detected substantial macrophage infiltration in the kidney. In a mouse model of rhabdomyolysis-induced AKI, diverse renal macrophage phenotypes were observed depending on the stage of the disease. Two days after rhabdomyolysis, F4/80(low)CD11b(high)Ly6b(high)CD206(low) kidney macrophages were dominant, whereas by day 8, F4/80(high)CD11b(+)Ly6b(low)CD206(high) cells became the most abundant. Single-cell gene expression analyses of FACS-sorted macrophages revealed that these subpopulations were heterogeneous and that individual cells simultaneously expressed both M1 and M2 markers. Liposomal clodronate-mediated macrophage depletion significantly reduced the early infiltration of F4/80(low)CD11b(high)Ly6b(high)CD206(low) macrophages. Furthermore, transcriptionally regulated targets potentially involved in disease progression, including fibronectin, collagen III, and chemoattractants that were identified via single-cell analysis, were verified as macrophage-dependent in situ. In vitro, myoglobin treatment induced proximal tubular cells to secrete chemoattractants and macrophages to express proinflammatory markers. At day 30, liposomal clodronate-mediated macrophage depletion reduced fibrosis and improved both kidney repair and mouse survival. Seven months after rhabdomyolysis, histologic lesions were still present but were substantially reduced with prior depletion of macrophages. These results suggest an important role for macrophages in rhabdomyolysis-induced AKI progression and advocate the utility of long-term follow-up for patients with this disease.
Keywords: Immunology and pathology; acute renal failure; chronic kidney disease; fibrosis; macrophages.; rhabdomyolysis.
Copyright © 2015 by the American Society of Nephrology.
Figures


References
-
- Bosch X, Poch E, Grau JM: Rhabdomyolysis and acute kidney injury. N Engl J Med 361: 62–72, 2009 - PubMed
-
- Zager RA: Studies of mechanisms and protective maneuvers in myoglobinuric acute renal injury. Lab Invest 60: 619–629, 1989 - PubMed
-
- Zager RA, Gamelin LM: Pathogenetic mechanisms in experimental hemoglobinuric acute renal failure. Am J Physiol 256: F446–F455, 1989 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

