Role of Porphyromonas gingivalis gingipains in multi-species biofilm formation
- PMID: 25270662
- PMCID: PMC4189655
- DOI: 10.1186/s12866-014-0258-7
Role of Porphyromonas gingivalis gingipains in multi-species biofilm formation
Abstract
Background: Periodontal diseases are polymicrobial diseases that cause the inflammatory destruction of the tooth-supporting (periodontal) tissues. Their initiation is attributed to the formation of subgingival biofilms that stimulate a cascade of chronic inflammatory reactions by the affected tissue. The Gram-negative anaerobes Porphyromonas gingivalis, Tannerella forsythia and Treponema denticola are commonly found as part of the microbiota of subgingival biofilms, and they are associated with the occurrence and severity of the disease. P. gingivalis expresses several virulence factors that may support its survival, regulate its communication with other species in the biofilm, or modulate the inflammatory response of the colonized host tissue. The most prominent of these virulence factors are the gingipains, which are a set of cysteine proteinases (either Arg-specific or Lys-specific). The role of gingipains in the biofilm-forming capacity of P. gingivalis is barely investigated. Hence, this in vitro study employed a biofilm model consisting of 10 "subgingival" bacterial species, incorporating either a wild-type P. gingivalis strain or its derivative Lys-gingipain and Arg-gingipan isogenic mutants, in order to evaluate quantitative and qualitative changes in biofilm composition.
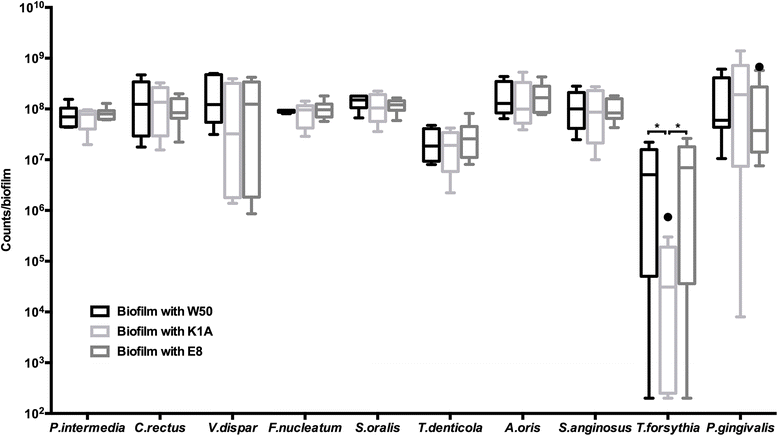
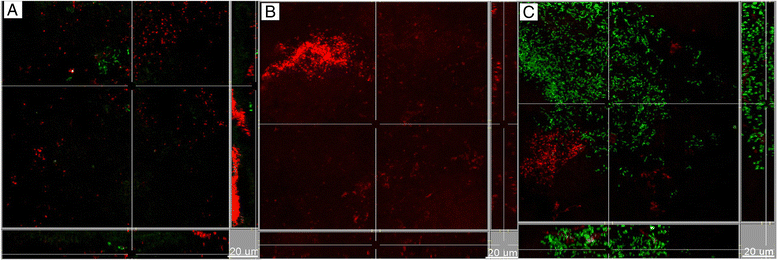
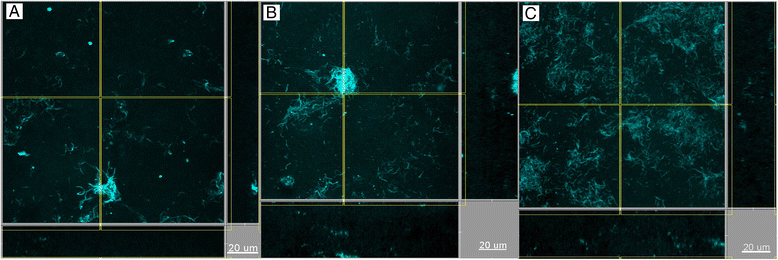
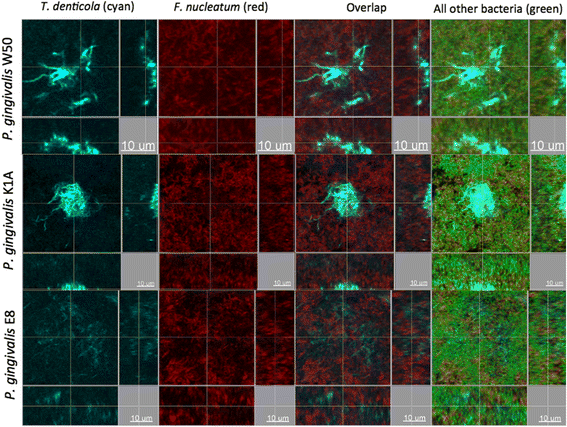
Results: Following 64 h of biofilm growth, the levels of all 10 species were quantified by fluorescence in situ hybridization or immunofluorescence. The wild-type and the two gingipain-deficient P. gingivalis strains exhibited similar growth in their corresponding biofilms. Among the remaining nine species, only the numbers of T. forsythia were significantly reduced, and only when the Lys-gingipain mutant was present in the biofilm. When evaluating the structure of the biofilm by confocal laser scanning microscopy, the most prominent observation was a shift in the spatial arrangement of T. denticola, in the presence of P. gingivalis Arg-gingipain mutant.
Conclusions: The gingipains of P. gingivalis may qualitatively and quantitatively affect composition of polymicrobial biofilms. The present experimental model reveals interdependency between the gingipains of P. gingivalis and T. forsythia or T. denticola.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

