Cell factories for insulin production
- PMID: 25270715
- PMCID: PMC4203937
- DOI: 10.1186/s12934-014-0141-0
Cell factories for insulin production
Abstract
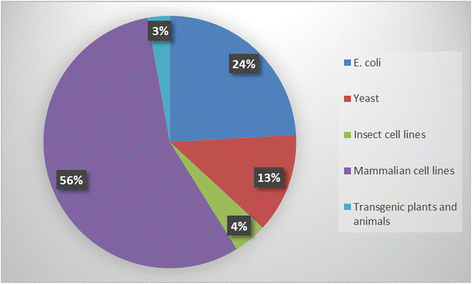
The rapid increase in the number of diabetic patients globally and exploration of alternate insulin delivery methods such as inhalation or oral route that rely on higher doses, is bound to escalate the demand for recombinant insulin in near future. Current manufacturing technologies would be unable to meet the growing demand of affordable insulin due to limitation in production capacity and high production cost. Manufacturing of therapeutic recombinant proteins require an appropriate host organism with efficient machinery for posttranslational modifications and protein refolding. Recombinant human insulin has been produced predominantly using E. coli and Saccharomyces cerevisiae for therapeutic use in human. We would focus in this review, on various approaches that can be exploited to increase the production of a biologically active insulin and its analogues in E. coli and yeast. Transgenic plants are also very attractive expression system, which can be exploited to produce insulin in large quantities for therapeutic use in human. Plant-based expression system hold tremendous potential for high-capacity production of insulin in very cost-effective manner. Very high level of expression of biologically active proinsulin in seeds or leaves with long-term stability, offers a low-cost technology for both injectable as well as oral delivery of proinsulin.
Figures
References
-
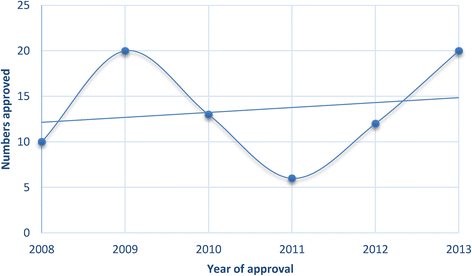
- Walsh G. Biopharmaceuticals: approvals and approval trends in 2004. Biopharm Int. 2005;18:58–65.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases