Risk of bias in industry-funded oseltamivir trials: comparison of core reports versus full clinical study reports
- PMID: 25270852
- PMCID: PMC4179405
- DOI: 10.1136/bmjopen-2014-005253
Risk of bias in industry-funded oseltamivir trials: comparison of core reports versus full clinical study reports
Abstract
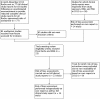
Background: The Cochrane risk of bias tool is a prominent instrument used to evaluate potential biases in clinical trials. In three updates of our Cochrane review on neuraminidase inhibitors, we assessed risk of bias on the same trials using different levels of detail: the trials in journal publications, in core reports, and in full clinical study reports. Here we analyse whether progressively greater amounts of information and detail in full clinical study reports (including trial protocols, statistical analysis plans, certificates of analyses, individual participant data listings and randomisation lists) affected our risk of bias assessments.
Methods and findings: We used the Cochrane risk of bias tool to assess and compare risk of bias in 14 oseltamivir trials (reported in 10 clinical study reports) obtained from the European Medicines Agency (EMA) and the manufacturer, Roche. With more detailed information, reported in clinical study reports, no previous assessment of 'high' risk of bias was reclassified as 'low' or 'unclear' in the main analysis, and over half (55%, 34/62) of the previous assessments of 'low' risk of bias were reclassified as 'high'. Most assessments of 'unclear' risk of bias (67%, or 28/42) were reclassified as 'high' risk of bias when our judgements were based on full clinical study reports. The limits of our study were our relative inexperience in dealing with large information sets, sometimes subjective bias judgements and focus on industry trials. Comparison with journal publications was not possible because of the low number of trials published.
Conclusions: We found that as information increased in the document, this increased our assessment of bias. This may mean that risk of bias has been insufficiently assessed in Cochrane reviews based on journal publications.
Keywords: STATISTICS & RESEARCH METHODS.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
References
-
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Structure and Content of Clinical Study Reports: E3 [Internet]. 1995[cited 8 July 2012]. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Eff...
-
- European Medicines Agency. European Medicines Agency policy on access to documents (related to medicinal products for human and veterinary use) POLICY/0043 [Internet]. 2010[cited 14 May 2012]. http://www.ema.europa.eu/docs/en_GB/document_library/Other/2010/11/WC500...
-
- F. Hoffmann-La Roche Ltd. Roche Global Policy on Sharing of Clinical Trials Data [Internet]. 2013. [cited 19 Jun 2013]. http://roche-trials.com/dataSharingPolicy.action
-
- Nisen P, Rockhold F. Access to patient-level data from GlaxoSmithKline clinical trials. N Engl J Med 2013;369:475–8 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials