Study protocol for a double blind, randomised, placebo-controlled trial of continuous subpectoral local anaesthetic infusion for pain and shoulder function following mastectomy: SUB-pectoral Local anaesthetic Infusion following MastEctomy (SUBLIME) study
- PMID: 25270861
- PMCID: PMC4179566
- DOI: 10.1136/bmjopen-2014-006318
Study protocol for a double blind, randomised, placebo-controlled trial of continuous subpectoral local anaesthetic infusion for pain and shoulder function following mastectomy: SUB-pectoral Local anaesthetic Infusion following MastEctomy (SUBLIME) study
Abstract
Introduction: Over 16 000 mastectomies are performed in England and Wales annually. Acute postoperative pain and nausea are common. The most frequently occurring long-term complications are chronic pain (up to 50%) and reduced shoulder function (reported at 35%). Regional techniques that improve acute postoperative pain relief may reduce the incidence of these complications. This study assesses the effectiveness of a 24-hour continuous local anaesthetic in the subpectoral plane in improving postoperative pain and quality of life in patients undergoing mastectomy.
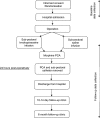
Methods and analysis: This is a randomised, double blind, placebo-controlled, two-centre, parallel group trial in women undergoing mastectomy with or without axillary involvement. One hundred and sixty participants will be randomised in a 1:1 ratio to receive either 0.25% levobupivacaine or 0.9% saline by subpectoral infusion postoperatively for 24 h. All participants will be provided with an intravenous morphine patient-controlled analgesia (PCA) system. Participants will be followed-up for 24 h in hospital and at approximately 14 days and 6 months postoperatively. Joint primary outcome measures are total morphine consumption and total pain score (captured via patient-recorded visual analogue scale (VAS) 4 hourly) during the first 24 h postoperatively. Primary statistical analysis of total pain is based on the area under the curve of pain versus time graph. Secondary outcomes include PCA attempts in first 24 h; VAS pain scores and shoulder function by goniometry at 24 h, 14 days (approximately) and 6 months; Verbal Rating Scale pain scores in first 24 h; Brief Pain Inventory and Oxford Shoulder Score at 6 months; duration of hospital stay; incidence of postoperative nausea and vomiting; cost-effectiveness.
Ethics and dissemination: The study is approved by the South West England Research Ethics Committee (12/SW/0149).
Results: will be published in a peer-reviewed journal and presented at local, national and international scientific meetings.
Trial registration: ISRCTN46621916. EudraCT 2011-005775-16.
Keywords: Anaesthetic infusion; Local Anaesthetic; Mastectomy; Pain; Shoulder function.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
References
-
- Statistical Information Team, Cancer Research UK, 2010
-
- The NHS Information Centre. National Mastectomy and Breast Reconstruction Audit 2011: A National Audit of the Provision and Outcomes of Mastectomy and Breast Reconstruction for Women in England. Fourth Annual Report 2011
-
- Health and Social Care Information Centre. Hospital Episode Statistics: main procedures and interventions 2012–2013. http://www.hesonline.nhs.uk
-
- Skov J, Kroner K, Krebs B, et al. . Pain and dysesthesias in the mastectomy scar. Ugeskr Laeger 1990;152:3081–4 Article in Danish - PubMed
-
- Tasmuth T, von Smitten K, Hietanen P, et al. . Pain and other symptoms after different treatment modalities of breast cancer. Am Onc 1995;6:453–9 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical