Defective macroautophagic turnover of brain lipids in the TgCRND8 Alzheimer mouse model: prevention by correcting lysosomal proteolytic deficits
- PMID: 25270989
- PMCID: PMC4240291
- DOI: 10.1093/brain/awu278
Defective macroautophagic turnover of brain lipids in the TgCRND8 Alzheimer mouse model: prevention by correcting lysosomal proteolytic deficits
Abstract
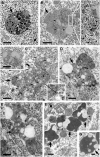
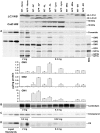
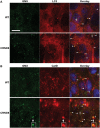
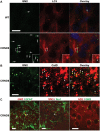
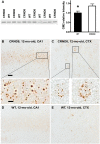
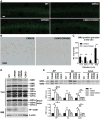
Autophagy, the major lysosomal pathway for the turnover of intracellular organelles is markedly impaired in neurons in Alzheimer's disease and Alzheimer mouse models. We have previously reported that severe lysosomal and amyloid neuropathology and associated cognitive deficits in the TgCRND8 Alzheimer mouse model can be ameliorated by restoring lysosomal proteolytic capacity and autophagy flux via genetic deletion of the lysosomal protease inhibitor, cystatin B. Here we present evidence that macroautophagy is a significant pathway for lipid turnover, which is defective in TgCRND8 brain where lipids accumulate as membranous structures and lipid droplets within giant neuronal autolysosomes. Levels of multiple lipid species including several sphingolipids (ceramide, ganglioside GM3, GM2, GM1, GD3 and GD1a), cardiolipin, cholesterol and cholesteryl esters are elevated in autophagic vacuole fractions and lysosomes isolated from TgCRND8 brain. Lipids are localized in autophagosomes and autolysosomes by double immunofluorescence analyses in wild-type mice and colocalization is increased in TgCRND8 mice where abnormally abundant GM2 ganglioside-positive granules are detected in neuronal lysosomes. Cystatin B deletion in TgCRND8 significantly reduces the number of GM2-positive granules and lowers the levels of GM2 and GM3 in lysosomes, decreases lipofuscin-related autofluorescence, and eliminates giant lipid-containing autolysosomes while increasing numbers of normal-sized autolysosomes/lysosomes with reduced content of undigested components. These findings have identified macroautophagy as a previously unappreciated route for delivering membrane lipids to lysosomes for turnover, a function that has so far been considered to be mediated exclusively through the endocytic pathway, and revealed that autophagic-lysosomal dysfunction in TgCRND8 brain impedes lysosomal turnover of lipids as well as proteins. The amelioration of lipid accumulation in TgCRND8 by removing cystatin B inhibition on lysosomal proteases suggests that enhancing lysosomal proteolysis improves the overall environment of the lysosome and its clearance functions, which may be possibly relevant to a broader range of lysosomal disorders beyond Alzheimer's disease.
Keywords: Alzheimer’s disease; TgCRND8; autophagy; lipids; lysosome.
© The Author (2014). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures

References
-
- Barrier L, Ingrand S, Damjanac M, Rioux Bilan A, Hugon J, Page G. Genotype-related changes of ganglioside composition in brain regions of transgenic mouse models of Alzheimer's disease. Neurobiol Aging. 2007;28:1863–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

