Accumulation of 5-hydroxynorvaline in maize (Zea mays) leaves is induced by insect feeding and abiotic stress
- PMID: 25271262
- PMCID: PMC4286406
- DOI: 10.1093/jxb/eru385
Accumulation of 5-hydroxynorvaline in maize (Zea mays) leaves is induced by insect feeding and abiotic stress
Abstract
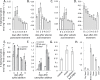
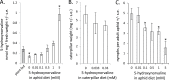
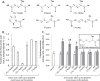
Plants produce a wide variety of defensive metabolites to protect themselves against herbivores and pathogens. Non-protein amino acids, which are present in many plant species, can have a defensive function through their mis-incorporation during protein synthesis and/or inhibition of biosynthetic pathways in primary metabolism. 5-Hydroxynorvaline was identified in a targeted search for previously unknown non-protein amino acids in the leaves of maize (Zea mays) inbred line B73. Accumulation of this compound increases during herbivory by aphids (Rhopalosiphum maidis, corn leaf aphid) and caterpillars (Spodoptera exigua, beet armyworm), as well as in response to treatment with the plant signalling molecules methyl jasmonate, salicylic acid and abscisic acid. In contrast, ethylene signalling reduced 5-hydroxynorvaline abundance. Drought stress induced 5-hydroxynorvaline accumulation to a higher level than insect feeding or treatment with defence signalling molecules. In field-grown plants, the 5-hydroxynorvaline concentration was highest in above-ground vegetative tissue, but it was also detectable in roots and dry seeds. When 5-hydroxynorvaline was added to aphid artificial diet at concentrations similar to those found in maize leaves and stems, R. maidis reproduction was reduced, indicating that this maize metabolite may have a defensive function. Among 27 tested maize inbred lines there was a greater than 10-fold range in the accumulation of foliar 5-hydroxynorvaline. Genetic mapping populations derived from a subset of these inbred lines were used to map quantitative trait loci for 5-hydroxynorvaline accumulation to maize chromosomes 5 and 7.
Keywords: 5-hydroxynorvaline; Aphid; Rhopalosiphum maidis.; drought; maize.
© The Author 2014. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures



References
-
- Ankala A, Luthe DS, Williams WP, Wilkinson JR. 2009. Integration of ethylene and jasmonic acid signaling pathways in the expression of maize defense protein Mir1-CP. Molecular Plant-Microbe Interactions 22, 1555–1564. - PubMed
-
- Bradbury PJ, Zhang Z, Kroon DE, Casstevens TM, Ramdoss Y, Buckler ES. 2007. TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics 23, 2633–2635. - PubMed
-
- Brown DH, Fowden L. 1966. Characterization of δ-acetyl-L-ornithine isolated from Onobrychis vicifolia Scop. Phytochemistry 5, 881–886.
-
- Buckler ES, Holland JB, Bradbury PJ, Acharya CB, Brown PJ, Browne C, Ersoz E, Flint-Garcia S, Garcia A, Glaubitz JC, et al. 2009. The genetic architecture of maize flowering time. Science 325, 714–718. - PubMed
-
- Carmo-Silva AE, Keys AJ, Beale MH, Ward JL, Baker JM, Hawkins ND, Arrabaca MC, Parry MA. 2009. Drought stress increases the production of 5-hydroxynorvaline in two C4 grasses. Phytochemistry 70, 664–671. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

