Endosomal generation of cAMP in GPCR signaling
- PMID: 25271346
- PMCID: PMC4417940
- DOI: 10.1038/nchembio.1611
Endosomal generation of cAMP in GPCR signaling
Abstract
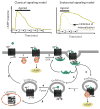
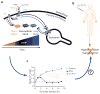
It has been widely assumed that the production of the ubiquitous second messenger cyclic AMP, which is mediated by cell surface G protein–coupled receptors (GPCRs), and its termination take place exclusively at the plasma membrane. Recent studies reveal that diverse GPCRs do not always follow this conventional paradigm. In the new model, GPCRs mediate G-protein signaling not only from the plasma membrane but also from endosomal membranes. This model proposes that following ligand binding and activation, cell surface GPCRs internalize and redistribute into early endosomes, where trimeric G protein signaling can be maintained for an extended period of time. This Perspective discusses the molecular and cellular mechanistic subtleties as well as the physiological consequences of this unexpected process, which is considerably changing how we think about GPCR signaling and regulation and how we study drugs that target this receptor family.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Lefkowitz RJ. Seven transmembrane receptors: something old, something new. Acta Physiol (Oxf) 2007;190:9–19. - PubMed
-
- Vilardaga JP, Bunemann M, Krasel C, Castro M, Lohse MJ. Measurement of the millisecond activation switch of G protein–coupled receptors in living cells. Nat Biotechnol. 2003;21:807–812. - PubMed
-
- Nikolaev VO, Hoffmann C, Bunemann M, Lohse MJ, Vilardaga JP. Molecular basis of partial agonism at the neurotransmitter a2A-adrenergic receptor and Gi-protein heterotrimer. J Biol Chem. 2006;281:24506–24511. - PubMed
-
- Lohse MJ, et al. Optical techniques to analyze real-time activation and signaling of G-protein–coupled receptors. Trends Pharmacol Sci. 2008;29:159–165. - PubMed
-
- Vilardaga JP, Romero G, Feinstein TN, Wehbi VL. Kinetics and dynamics in the G protein–coupled receptor signaling cascade. Methods Enzymol. 2013;522:337–363. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

