Tempol, a superoxide dismutase mimetic agent, ameliorates cisplatin-induced nephrotoxicity through alleviation of mitochondrial dysfunction in mice
- PMID: 25271439
- PMCID: PMC4182751
- DOI: 10.1371/journal.pone.0108889
Tempol, a superoxide dismutase mimetic agent, ameliorates cisplatin-induced nephrotoxicity through alleviation of mitochondrial dysfunction in mice
Erratum in
- PLoS One. 2014;9(12):e115983
Abstract
Background: Mitochondrial dysfunction is a crucial mechanism by which cisplatin, a potent chemotherapeutic agent, causes nephrotoxicity where mitochondrial electron transport complexes are shifted mostly toward imbalanced reactive oxygen species versus energy production. In the present study, the protective role of tempol, a membrane-permeable superoxide dismutase mimetic agent, was evaluated on mitochondrial dysfunction and the subsequent damage induced by cisplatin nephrotoxicity in mice.
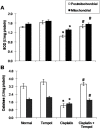
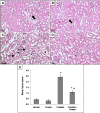
Methods and findings: Nephrotoxicity was assessed 72 h after a single i.p. injection of cisplatin (25 mg/kg) with or without oral administration of tempol (100 mg/kg/day). Serum creatinine and urea as well as glucosuria and proteinuria were evaluated. Both kidneys were isolated for estimation of oxidative stress markers, adenosine triphosphate (ATP) content and caspase-3 activity. Moreover, mitochondrial oxidative phosphorylation capacity, complexes I-IV activities and mitochondrial nitric oxide synthase (mNOS) protein expression were measured along with histological examinations of renal tubular damage and mitochondrial ultrastructural changes. Tempol was effective against cisplatin-induced elevation of serum creatinine and urea as well as glucosuria and proteinuria. Moreover, pretreatment with tempol notably inhibited cisplatin-induced oxidative stress and disruption of mitochondrial function by restoring mitochondrial oxidative phosphorylation, complexes I and III activities, mNOS protein expression and ATP content. Tempol also provided significant protection against apoptosis, tubular damage and mitochondrial ultrastructural changes. Interestingly, tempol did not interfere with the cytotoxic effect of cisplatin against the growth of solid Ehrlich carcinoma.
Conclusion: This study highlights the potential role of tempol in inhibiting cisplatin-induced nephrotoxicity without affecting its antitumor activity via amelioration of oxidative stress and mitochondrial dysfunction.
Conflict of interest statement
Figures



References
-
- Langerak AD, Dreisbach LP (2001) Chemotherapy regimens and cancer care. Texas: Landes Bioscience; Georgetown.
-
- Sahni V, Choudhury D, Ahmed Z (2009) Chemotherapy-associated renal dysfunction. Nat Rev Nephrol 8: 450–462. - PubMed
-
- Yao X, Panichpisal K, Kurtzman N, Nugent K (2007) Cisplatin nephrotoxicity: a review. Am J Med Sci 334: 115–124. - PubMed
-
- Pabla N, Dong Z (2008) Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int 73: 994–1007. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

