Biomaterial-based delivery for skeletal muscle repair
- PMID: 25271446
- PMCID: PMC4377112
- DOI: 10.1016/j.addr.2014.09.008
Biomaterial-based delivery for skeletal muscle repair
Abstract
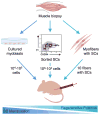
Skeletal muscle possesses a remarkable capacity for regeneration in response to minor damage, but severe injury resulting in a volumetric muscle loss can lead to extensive and irreversible fibrosis, scarring, and loss of muscle function. In early clinical trials, the intramuscular injection of cultured myoblasts was proven to be a safe but ineffective cell therapy, likely due to rapid death, poor migration, and immune rejection of the injected cells. In recent years, appropriate therapeutic cell types and culturing techniques have improved progenitor cell engraftment upon transplantation. Importantly, the identification of several key biophysical and biochemical cues that synergistically regulate satellite cell fate has paved the way for the development of cell-instructive biomaterials that serve as delivery vehicles for cells to promote in vivo regeneration. Material carriers designed to spatially and temporally mimic the satellite cell niche may be of particular importance for the complete regeneration of severely damaged skeletal muscle.
Keywords: Cell therapy; Drug delivery; Microenvironmental cue; Muscle regeneration; Satellite cell; Synthetic niche; Tissue engineered muscle.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures

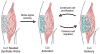

References
-
- Turner NJ, Badylak SF. Regeneration of skeletal muscle. Cell Tissue Res. 2012;347:759–774. - PubMed
-
- Jarvinen TAHJ, Jarvinen TLN, Kaariainen M, Kalimo A, Jarvinen M. Muscle injuries - Biology and treatment. Am J Sports Med. 2005;33:745–764. - PubMed
-
- Tedesco FS, Cossu G. Stem cell therapies for muscle disorders. Curr Opin Neurol. 2012;25:597–603. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

