Phrenic nerve stimulation increases human diaphragm fiber force after cardiothoracic surgery
- PMID: 25271750
- PMCID: PMC4299610
- DOI: 10.1164/rccm.201405-0993LE
Phrenic nerve stimulation increases human diaphragm fiber force after cardiothoracic surgery
Figures
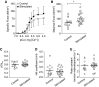
References
-
- Jaber S, Petrof BJ, Jung B, Chanques G, Berthet JP, Rabuel C, Bouyabrine H, Courouble P, Koechlin-Ramonatxo C, Sebbane M, et al. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med. 2011;183:364–371. - PubMed
-
- Welvaart WN, Paul MA, Stienen GJ, van Hees HW, Loer SA, Bouwman R, Niessen H, de Man FS, Witt CC, Granzier H, et al. Selective diaphragm muscle weakness after contractile inactivity during thoracic surgery. Ann Surg. 2011;254:1044–1049. - PubMed
-
- Powers SK, Wiggs MP, Sollanek KJ, Smuder AJ. Ventilator-induced diaphragm dysfunction: cause and effect. Am J Physiol Regul Integr Comp Physiol. 2013;305:R464–R477. - PubMed
-
- Masmoudi H, Coirault C, Demoule A, Mayaux J, Beuvin M, Romero N, Assouad J, Similowski T. Can phrenic stimulation protect the diaphragm from mechanical ventilation-induced damage? Eur Respir J. 2013;42:280–283. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

